New systemic anticancer treatments for gastric oesophageal cancer






- Slides: 33

New systemic anti-cancer treatments for gastric & oesophageal cancer: targeting immune functions Dr Simon Lord Consultant in Medical Oncology Early Phase Clinical Trials Unit, University of Oxford


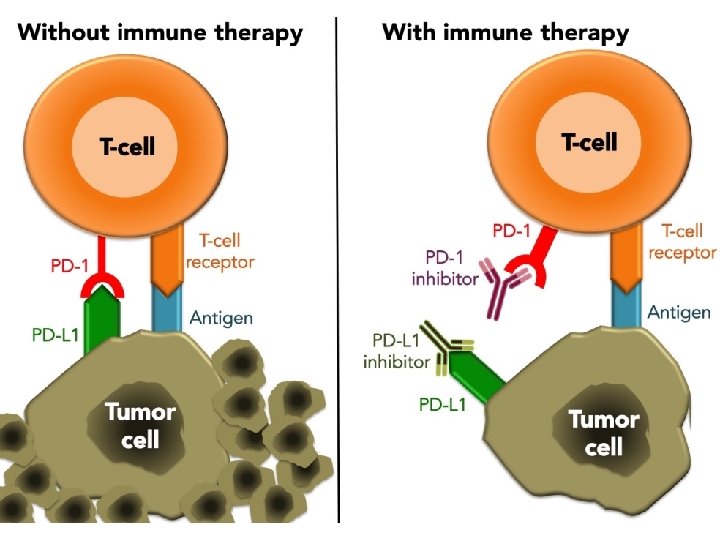
The immune system and cancer

Paul Ehrlich (1909) • First to put forward the concept of cancer immunosurveillance • Predicted that cancer would occur at ‘incredible frequency‘ if host defences did not prevent the outgrowth of continuously arising cancer cells

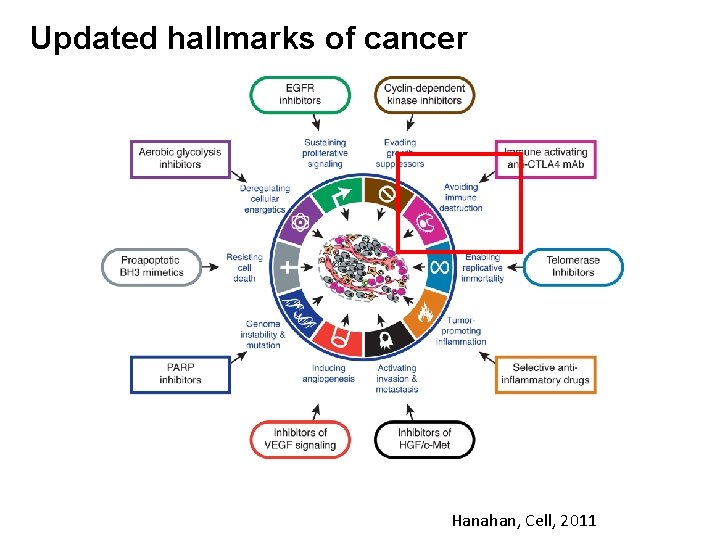
Updated hallmarks of cancer Hanahan, Cell, 2011

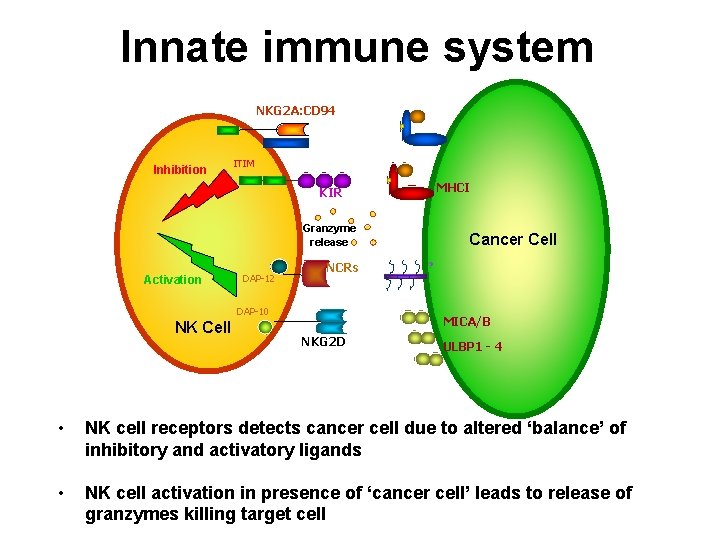
Innate immune system NKG 2 A: CD 94 Inhibition ITIM MHCI KIR Granzyme release Activation DAP-12 NCRs DAP-10 NK Cell Cancer Cell ? MICA/B NKG 2 D ULBP 1 - 4 • NK cell receptors detects cancer cell due to altered ‘balance’ of inhibitory and activatory ligands • NK cell activation in presence of ‘cancer cell’ leads to release of granzymes killing target cell

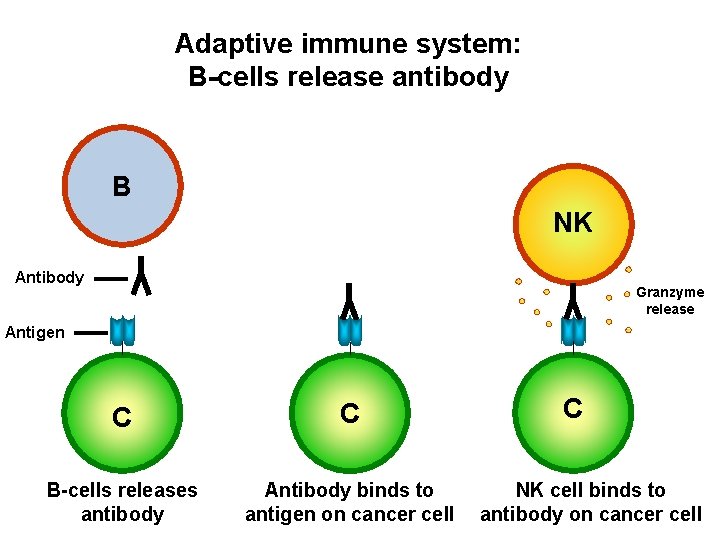
Adaptive immune system: B-cells release antibody B NK Antibody Granzyme release Antigen C C B-cells releases antibody Antibody binds to antigen on cancer cell C NK cell binds to antibody on cancer cell

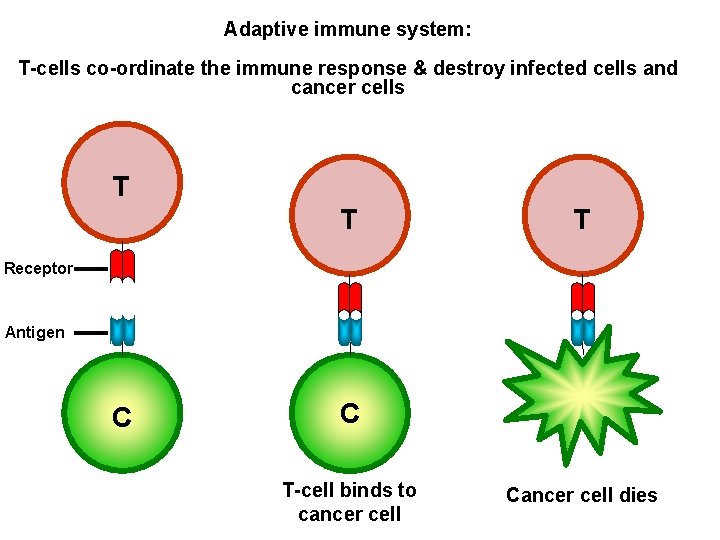
Adaptive immune system: T-cells co-ordinate the immune response & destroy infected cells and cancer cells T T T Receptor Antigen C C T-cell binds to cancer cell Cancer cell dies


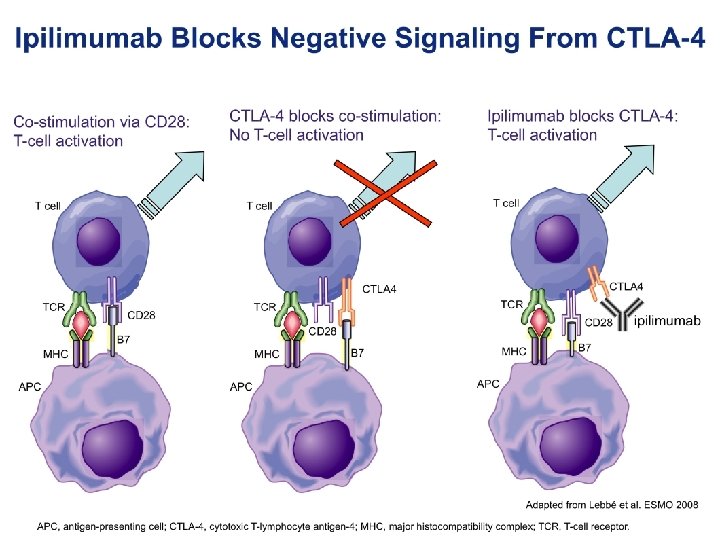
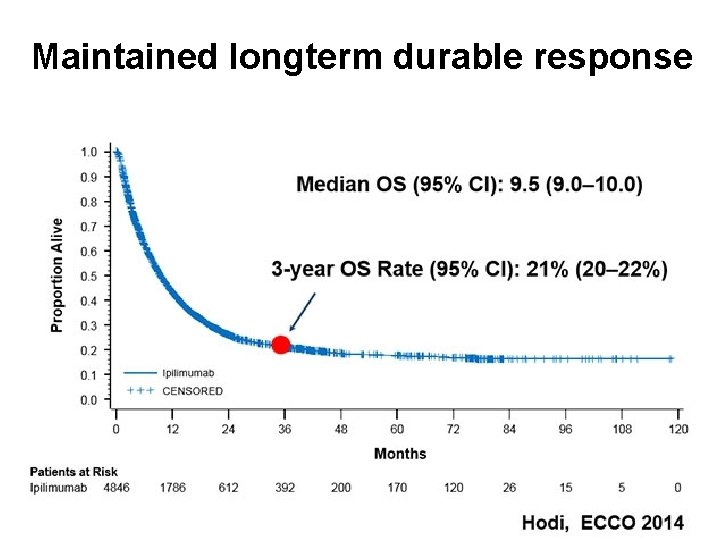
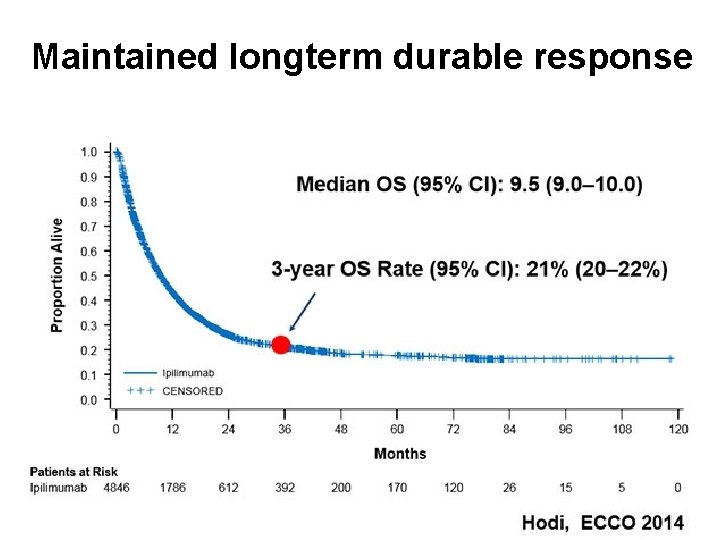
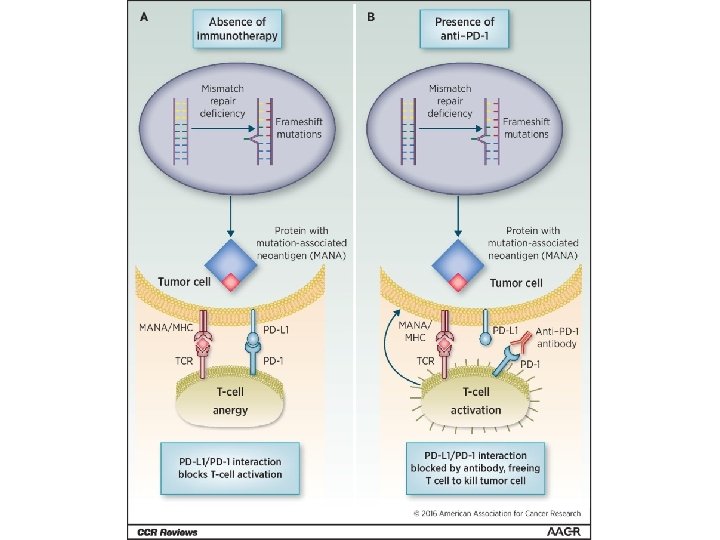
Maintained longterm durable response Immunotherapy: what is it and how does it work?

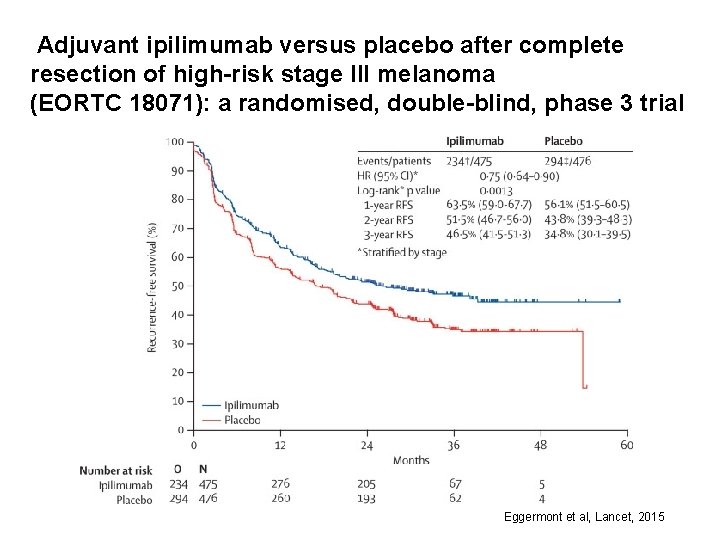
Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial Eggermont et al, Lancet, 2015


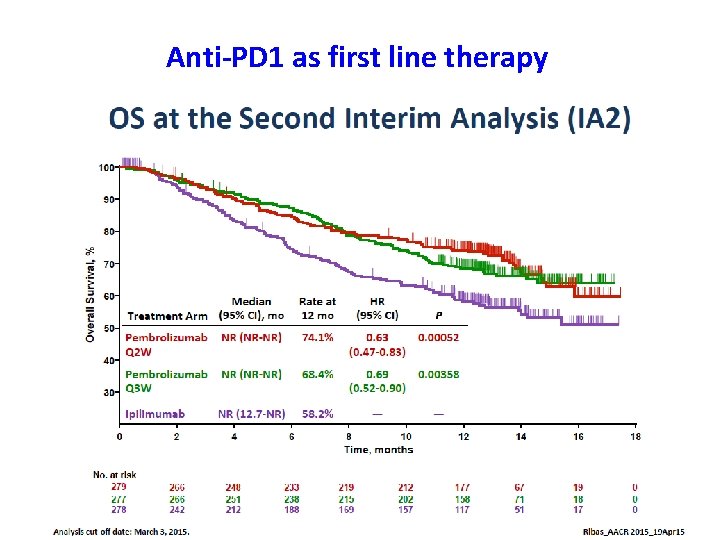
Anti-PD 1 as first line therapy

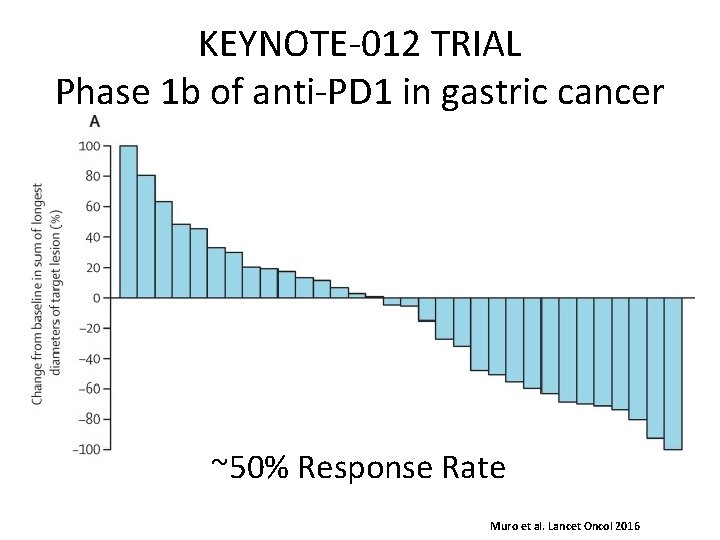
KEYNOTE-012 TRIAL Phase 1 b of anti-PD 1 in gastric cancer ~50% Response Rate Muro et al. Lancet Oncol 2016

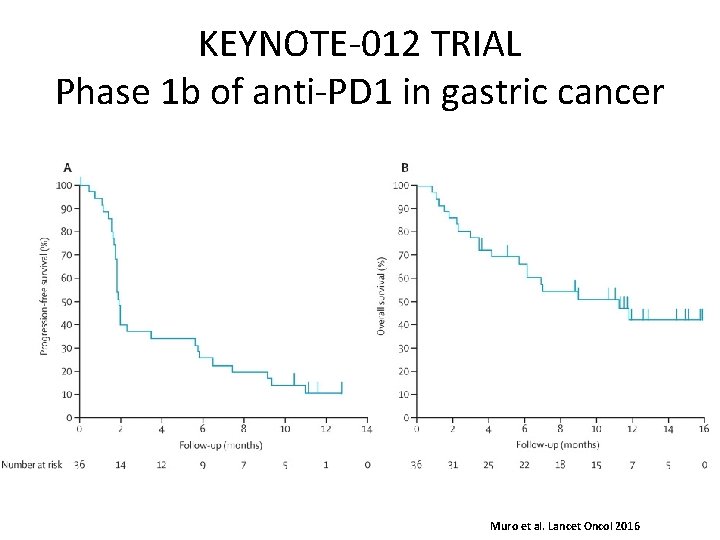
KEYNOTE-012 TRIAL Phase 1 b of anti-PD 1 in gastric cancer Muro et al. Lancet Oncol 2016

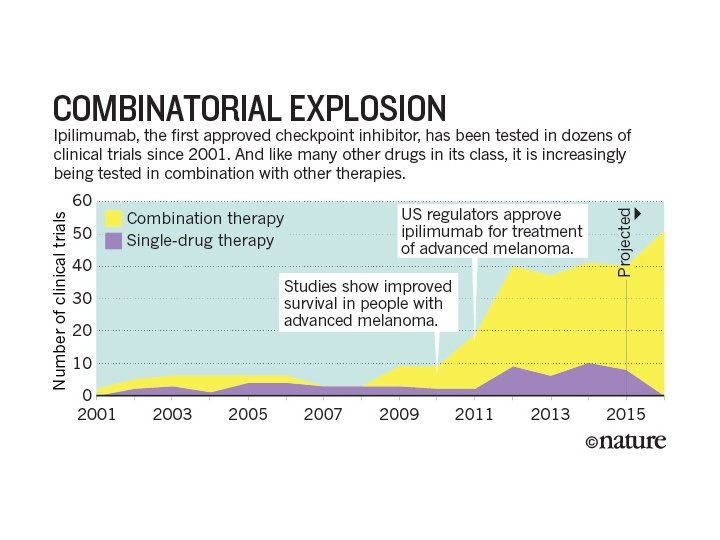
Combination treatment

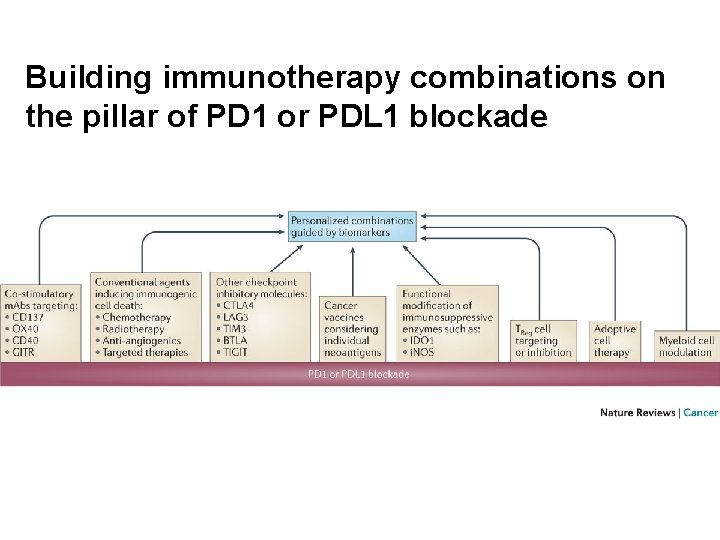
Building immunotherapy combinations on the pillar of PD 1 or PDL 1 blockade


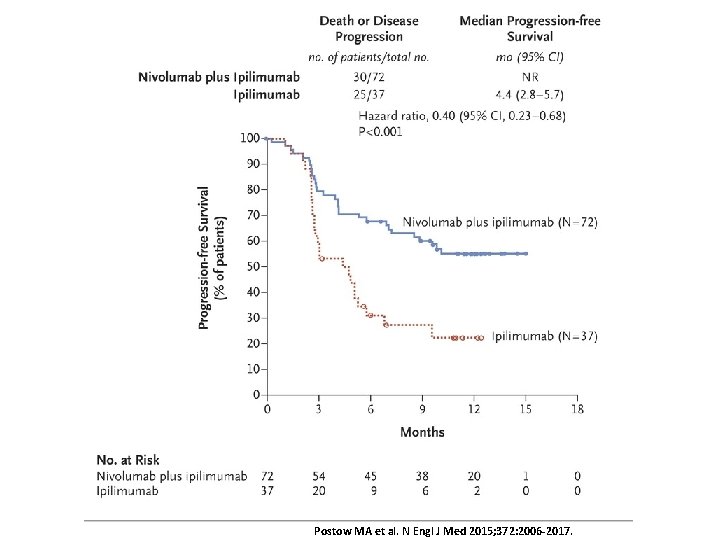
Postow MA et al. N Engl J Med 2015; 372: 2006 -2017.

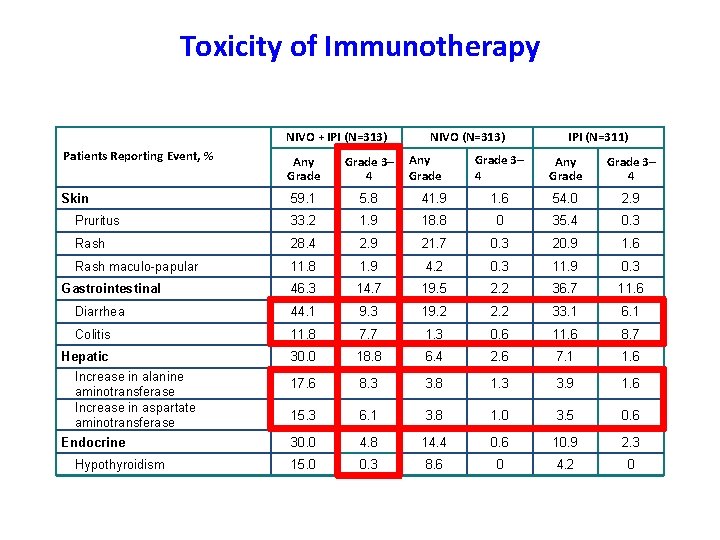
Toxicity of Immunotherapy NIVO + IPI (N=313) Patients Reporting Event, % Any Grade 3– 4 Skin 59. 1 5. 8 Pruritus 33. 2 Rash maculo-papular NIVO (N=313) Grade 3– 4 Any Grade 3– 4 41. 9 1. 6 54. 0 2. 9 18. 8 0 35. 4 0. 3 28. 4 2. 9 21. 7 0. 3 20. 9 1. 6 11. 8 1. 9 4. 2 0. 3 11. 9 0. 3 46. 3 14. 7 19. 5 2. 2 36. 7 11. 6 Diarrhea 44. 1 9. 3 19. 2 2. 2 33. 1 6. 1 Colitis 11. 8 7. 7 1. 3 0. 6 11. 6 8. 7 30. 0 18. 8 6. 4 2. 6 7. 1 1. 6 17. 6 8. 3 3. 8 1. 3 3. 9 1. 6 15. 3 6. 1 3. 8 1. 0 3. 5 0. 6 30. 0 4. 8 14. 4 0. 6 10. 9 2. 3 15. 0 0. 3 8. 6 0 4. 2 0 Gastrointestinal Hepatic Increase in alanine aminotransferase Increase in aspartate aminotransferase Endocrine Hypothyroidism Any Grade IPI (N=311)

Selecting patients

Maintained longterm durable response Immunotherapy: what is it and how does it work?

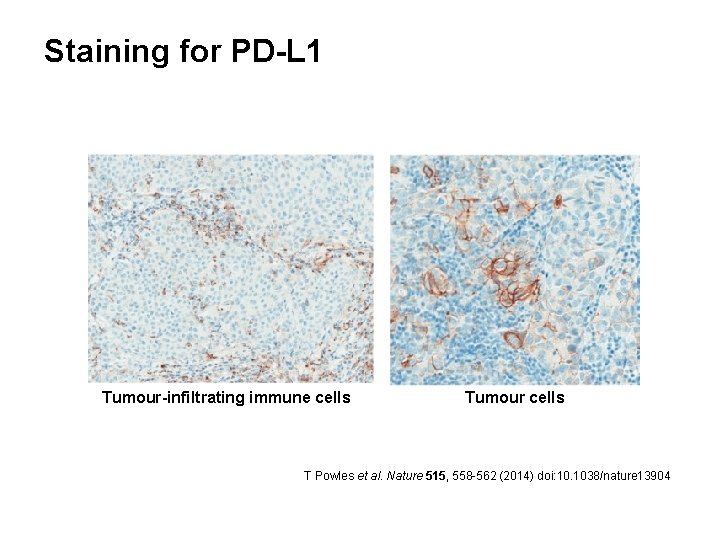
Staining for PD-L 1 Tumour-infiltrating immune cells Tumour cells T Powles et al. Nature 515, 558 -562 (2014) doi: 10. 1038/nature 13904

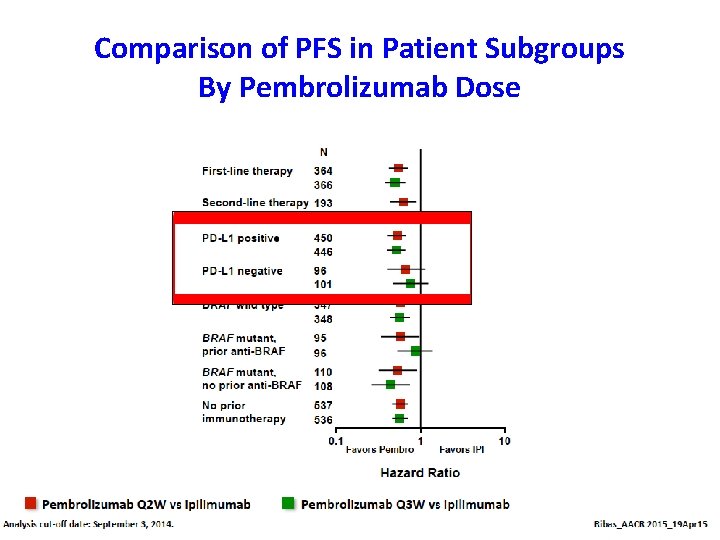
Comparison of PFS in Patient Subgroups By Pembrolizumab Dose

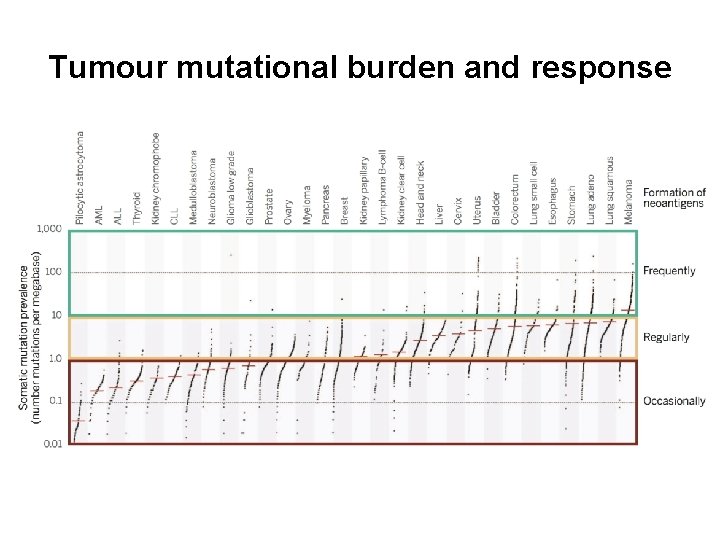
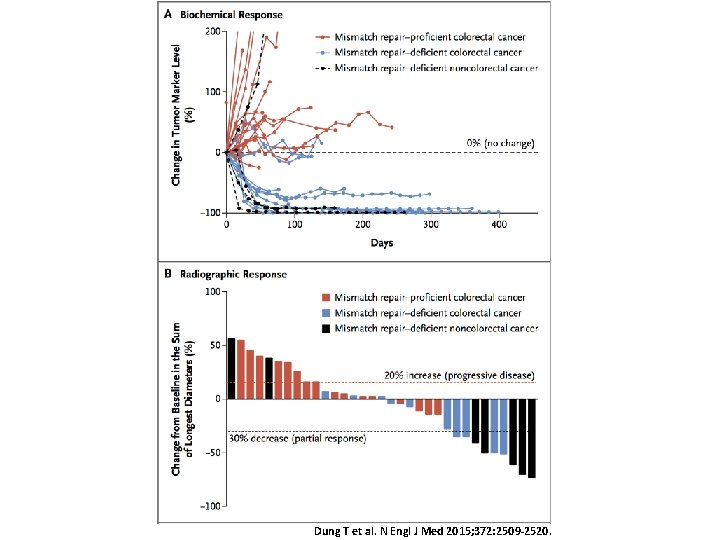
Tumour mutational burden and response


Dung T et al. N Engl J Med 2015; 372: 2509 -2520.

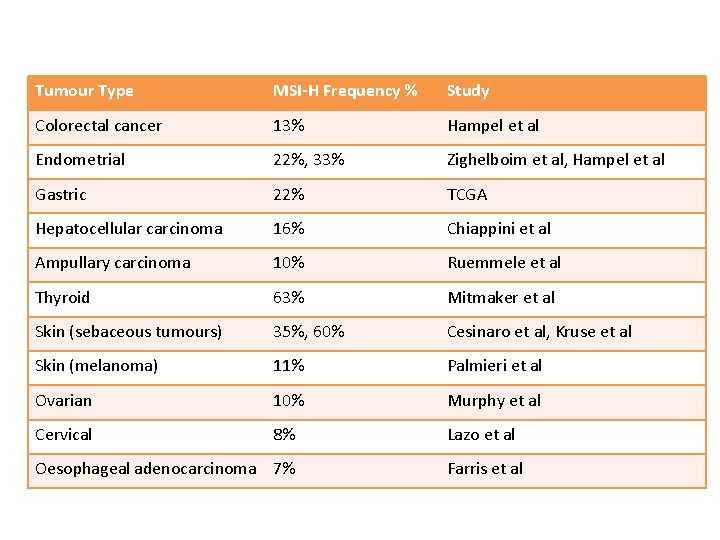
Tumour Type MSI-H Frequency % Study Colorectal cancer 13% Hampel et al Endometrial 22%, 33% Zighelboim et al, Hampel et al Gastric 22% TCGA Hepatocellular carcinoma 16% Chiappini et al Ampullary carcinoma 10% Ruemmele et al Thyroid 63% Mitmaker et al Skin (sebaceous tumours) 35%, 60% Cesinaro et al, Kruse et al Skin (melanoma) 11% Palmieri et al Ovarian 10% Murphy et al Cervical 8% Lazo et al Oesophageal adenocarcinoma 7% Farris et al

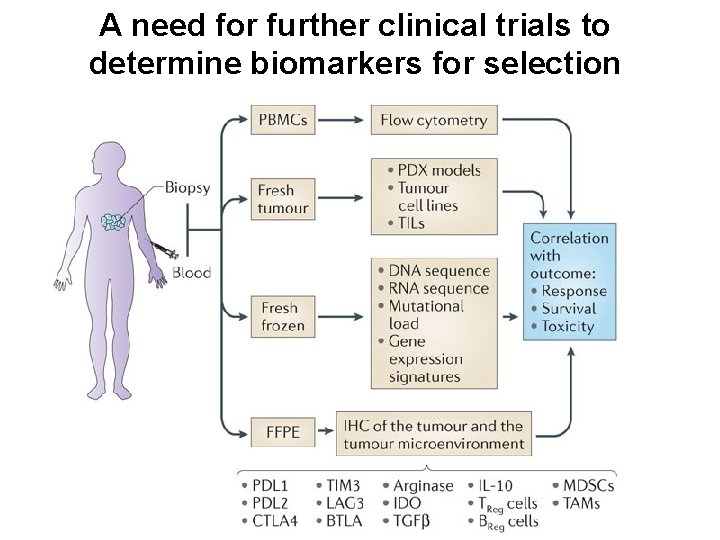
A need for further clinical trials to determine biomarkers for selection

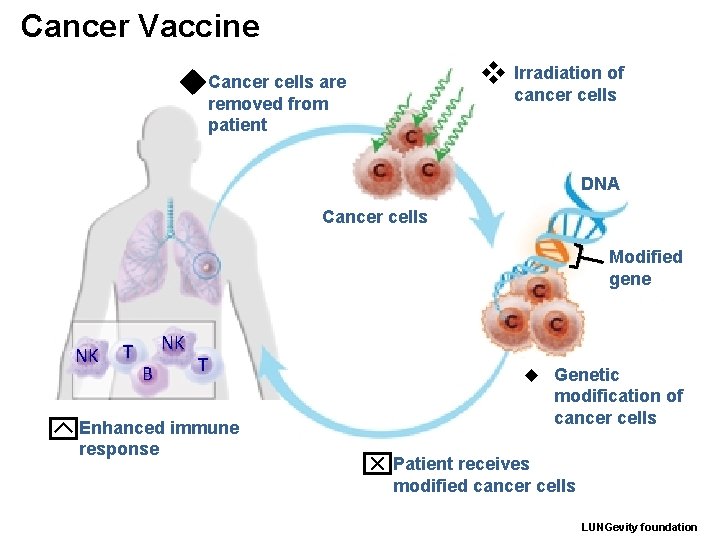
Cancer Vaccine v Irradiation of u Cancer cells are removed from patient cancer cells DNA Cancer cells Modified gene NK T NK B T immune y. Enhanced response w Genetic modification of cancer cells x. Patient receives modified cancer cells LUNGevity foundation

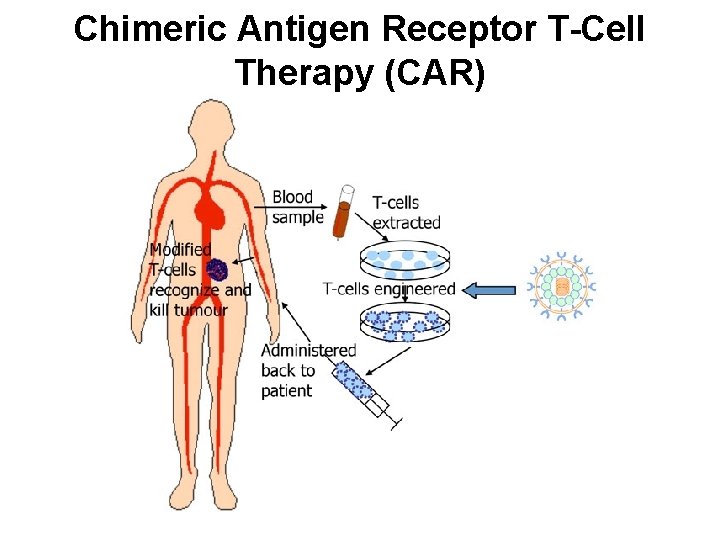
Chimeric Antigen Receptor T-Cell Therapy (CAR)

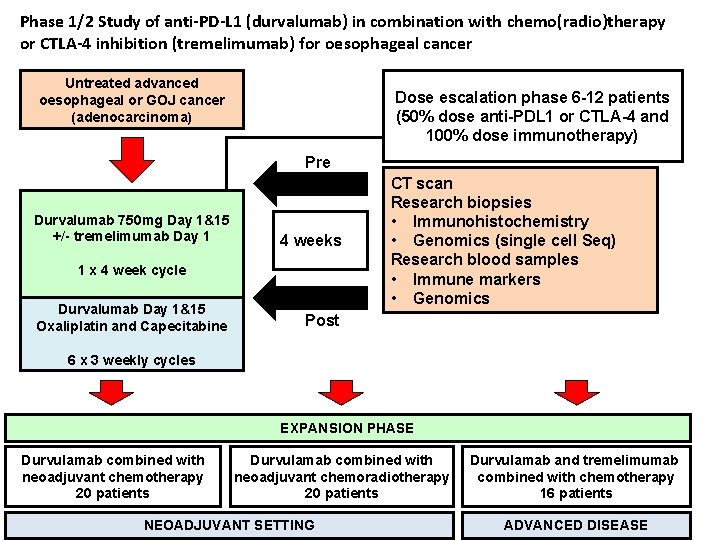
Phase 1/2 Study of anti-PD-L 1 (durvalumab) in combination with chemo(radio)therapy or CTLA-4 inhibition (tremelimumab) for oesophageal cancer Untreated advanced oesophageal or GOJ cancer (adenocarcinoma) Dose escalation phase 6 -12 patients (50% dose anti-PDL 1 or CTLA-4 and 100% dose immunotherapy) Pre Durvalumab 750 mg Day 1&15 +/- tremelimumab Day 1 4 weeks 1 x 4 week cycle Durvalumab Day 1&15 Oxaliplatin and Capecitabine CT scan Research biopsies • Immunohistochemistry • Genomics (single cell Seq) Research blood samples • Immune markers • Genomics Post 6 x 3 weekly cycles EXPANSION PHASE Durvulamab combined with neoadjuvant chemotherapy 20 patients Durvulamab combined with neoadjuvant chemoradiotherapy 20 patients NEOADJUVANT SETTING Durvulamab and tremelimumab combined with chemotherapy 16 patients ADVANCED DISEASE

Any queries about patients: Simon. lord@oncology. ox. ac. uk