New STA process Technical Engagement Jenna Dilkes Programme








- Slides: 11

New STA process Technical Engagement Jenna Dilkes Programme Manager - Planning and Operations, Technology Appraisals © NICE 2018. All rights reserved. Subject to notice of rights.

Technical Engagement – Who? What? Why? When? • Update on new Single Technology Appraisal (STA) process • When and why have we included Technical Engagement • What is it? • How will it look? • How can stakeholders get involved? • Post Technical Engagement actions 2

New Single Technology Appraisal Process (STA) • Introduced in April 2018 https: //www. nice. org. uk/process/pmg 19/chapter/ac knowledgements • Getting to the right decision at the right time 3

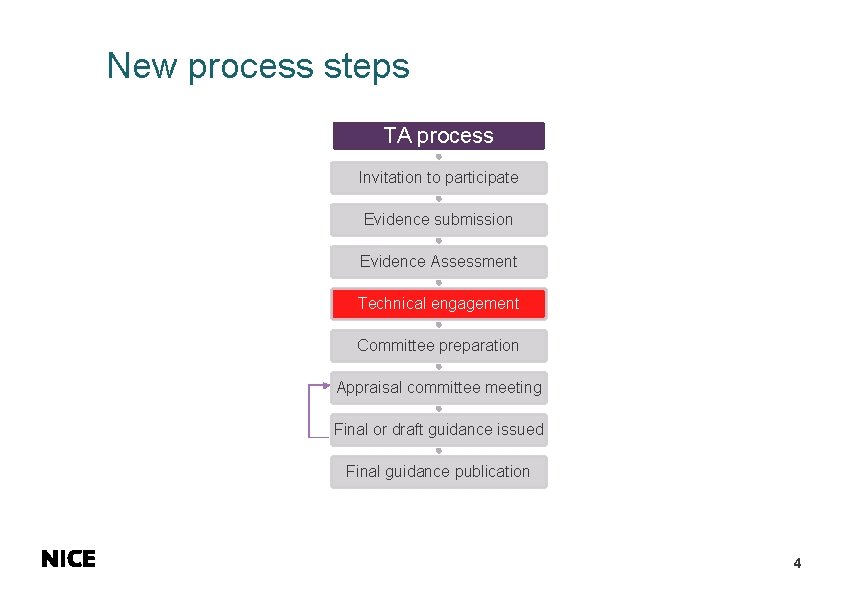
New process steps TA process Invitation to participate Evidence submission Evidence Assessment Technical engagement Committee preparation Appraisal committee meeting Final or draft guidance issued Final guidance publication 4

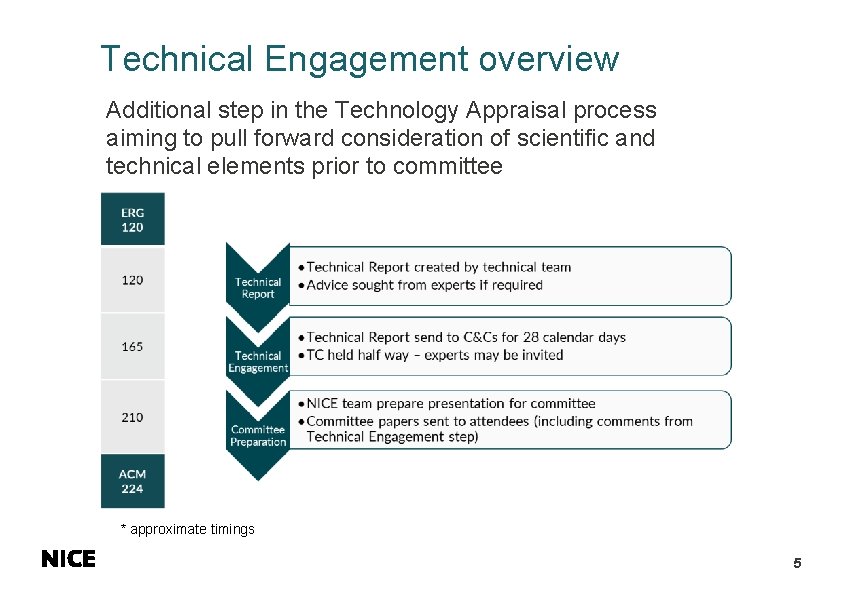
Technical Engagement overview Additional step in the Technology Appraisal process aiming to pull forward consideration of scientific and technical elements prior to committee * approximate timings 5

Technical Report example Issue # Stopping rule Background/description The trial protocol specified that treatment with the intervention should stop after 1 of issue year. The company (link to section of submission) assumed in their model that treatment stopped after 1 year to reflect the trial protocol. The technical team are concerned that in clinical practice, treatment may continue beyond 1 year, because the risk of relapse continues. Why this issue is important The stopping rule has a large impact on the cost effectiveness results, because there are no costs associated with the intervention after 1 year. A scenario analysis without the stopping rule increases the ICER from £ 45 k to £ 60 k. Technical team preliminary scientific judgement and rationale A 1 year stopping rule is not appropriate, because the risk of relapse remains, and it is unclear whether it would be possible to implement such a rule in clinical practice. Questions for engagement 1. In clinical practice, would treatment continue beyond 1 year? 2. Is it possible to implement a 1 year stopping rule in the NHS? 6

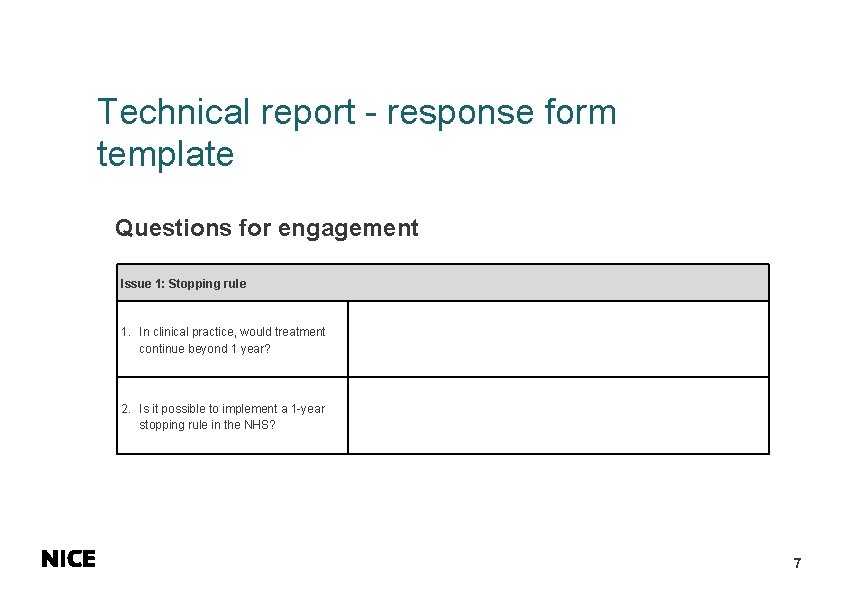
Technical report - response form template Questions for engagement Issue 1: Stopping rule 1. In clinical practice, would treatment continue beyond 1 year? 2. Is it possible to implement a 1 -year stopping rule in the NHS? 7

How do patient organisations get involved? Experts • Creation of the Technical Report (if required) • Technical engagement TC Stakeholder organisations • Responding to Technical Report questions during the engagement stage 8

Post Technical Engagement • NICE technical team update the technical report • The updated technical report and the technical engagement comments are included in the committee papers (sent 2 weeks prior to the appraisal committee meeting (ACm)) • Committee papers are also published on the NICE website post ACm alongside the ACD or FAD. 9

New process topics ID 1175 Durvalumab for maintenance treatment of unresectable non-small-cell lung cancer after platinum-based chemoradiation - Committee D Technical Engagement starts w/c 17. 12. 18. Committee meeting: 14. 02. 19 ID 1302 Osimertinib for untreated EGFR-positive non-small-cell lung cancer Committee D Technical Engagement starts w/c 14. 01. 19. Committee meeting: 20. 03. 19 ID 1318 Ribociclib in combination with fulvestrant for treating advanced hormone-receptor positive, HER 2 -negative breast cancer – Committee A Technical Engagement starts w/c 07. 01. 19. Committee meeting: 12. 03. 19 10

Questions? 11