New Measurement Unit The Mole 3 mathematical definitions















- Slides: 20


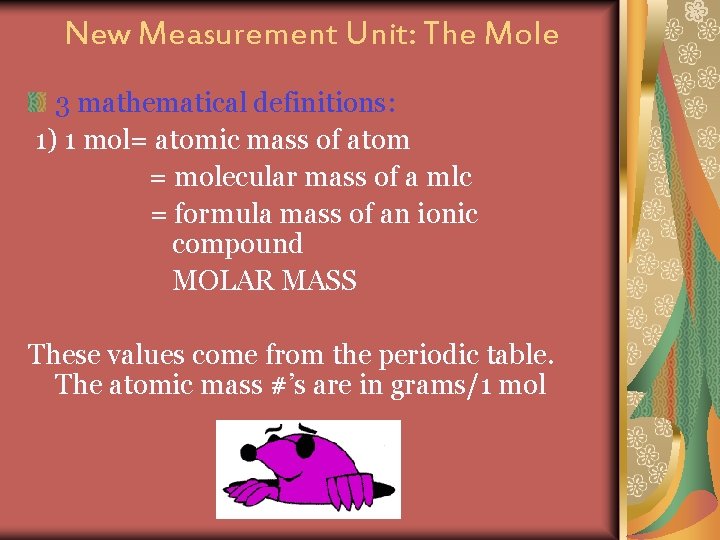
New Measurement Unit: The Mole 3 mathematical definitions: 1) 1 mol= atomic mass of atom = molecular mass of a mlc = formula mass of an ionic compound MOLAR MASS These values come from the periodic table. The atomic mass #’s are in grams/1 mol

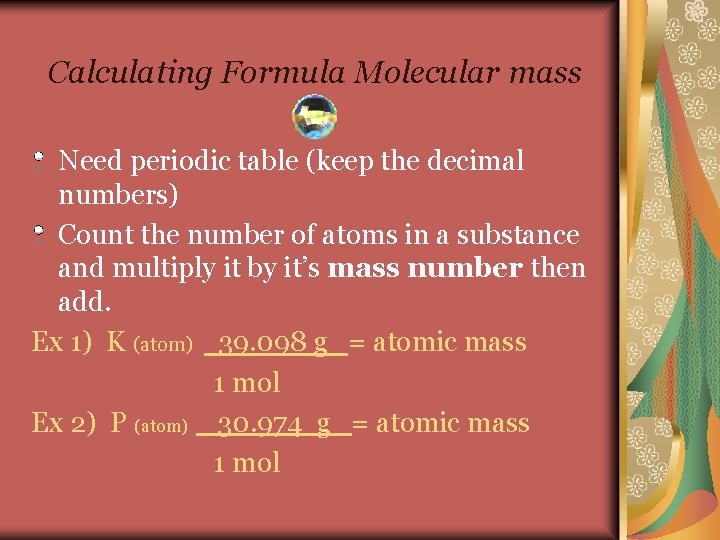
Calculating Formula Molecular mass Need periodic table (keep the decimal numbers) Count the number of atoms in a substance and multiply it by it’s mass number then add. Ex 1) K (atom) 39. 098 g = atomic mass 1 mol Ex 2) P (atom) 30. 974 g = atomic mass 1 mol

Ex 3) Potassium Chloride - KCl (formula unit) 1 K x 39. 098 1 Cl x 35. 453 = 74. 551 g/mol 74. 551 Ex 4) Magnesium Chloride - Mg. Cl 2 1 Mg x 24. 305 = 24. 305 2 Cl x 35. 453 = 70. 906 95. 211 g/mol Ex 5) Ammonium Carbonate - (NH 4)2 CO 3 2 N = 28. 02 8 H = 8. 08 1 C = 12. 01 3 O = 48. 00 = 96. 11 g/mol

2) 1 mol= 6. 02 x 1023 particles (avagadro’s number) where a “particle” can be: a) molecule b) atom c) ion d) formula unit of ionic cmpd 3) 1 mol= 22. 4 L for gas molecules

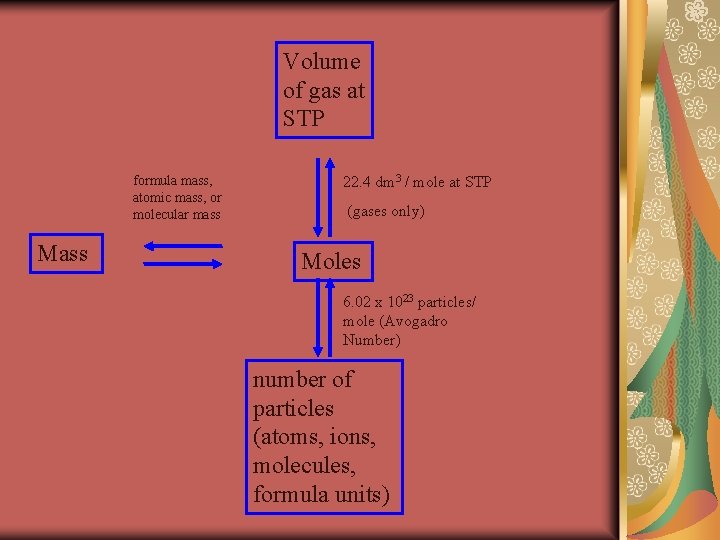
Volume of gas at STP formula mass, atomic mass, or molecular mass Mass 22. 4 dm 3 / mole at STP (gases only) Moles 6. 02 x 1023 particles/ mole (Avogadro Number) number of particles (atoms, ions, molecules, formula units)

One-Step Conversion Problems Use road maps to convert between one unit of chemical quantity to another. Dimensional Analysis – like metrics Use three definitions of a mole; an extension of the definitions in numerical form.

One-Step Conversion Problems These are your conversions factors! 1) 1 mole or x grams 1 mole 2) 1 mol or 6. 02 x 1023 particles 3) 1 mol 22. 4 L or 6. 02 x 1023 particles 1 mole 22. 4 L 1 mol GASES ONLY!

One- Step Conversions Ex 1) Convert 4. 3 grams of Na. Cl to moles. Mass mol Given: Unk: 4. 3 g Na. Cl x 1 mol Na. Cl = 7. 4 x 10 -2 mol Na. Cl 58. 45 g Na. Cl Ex 2) Convert 0. 00563 mol NH 3 to grams. Mol -> mass G: U: 0. 00563 mol NH 3 x 17 g NH 3 =9. 57 x 10 – 2 g NH 3 1 mol NH 3

More One-Steppers Ex) Given: 0. 91 Mol Na. Cl. O 3 Unk: ? Formula Units Na. Cl. O 3 Ex) Given 22. 92 x 1018 mlcs Cl 2 Unk: ? Mol Cl 2

More One-Steppers Ex) Given: 460. Mol Cl 2 gas Unk: ? L Cl 2 Ex) Given 84. 56 L Cl 2 Unk: ? Mol Cl 2


TWO –STEP CONVERSIONS Ex 1) Given: 8. 631 x 1021 atoms Na Unkn: ? g Na

TWO –STEP CONVERSIONS Ex 2) Given: 1. 5 x 1022 f. u. Mg. Cl 2 Unkn: ? g Mg. Cl 2

TWO –STEP CONVERSIONS Ex 3) Given: 2. 63 L O 2 Unkn: ? mg O 2

% Composition Definition: the % (in mass) of each element in a compound % mass element = g element x 100% g compound Ex An 8. 40 g sample of flourine completely combines with a 4. 90 g sample of sodium. Calculate the % composition of the compound that forms. mass element 1 + mass element 2 = total; 8. 40 g F 2 + 4. 90 g Na = 13. 30 g Na. F %F: 8. 40 g x 100 = 63. 2% F 13. 30 g %Na: 4. 90 g x 100 = 36. 8% Na 13. 30 g

% mass Cu. SO 4 1 Cu x 63. 546 g = 63. 546 g 1 S x 32. 066 g = 32. 066 g 4 O x 15. 999 g = 63. 996 g x 159. 608 g % Cu= 63. 546 x 100 = 39. 8% 159. 608 % S= 32. 066 x 100 = 20. 1% 159. 608 %O= 63. 996 x 100 = 40. 1% 159. 608

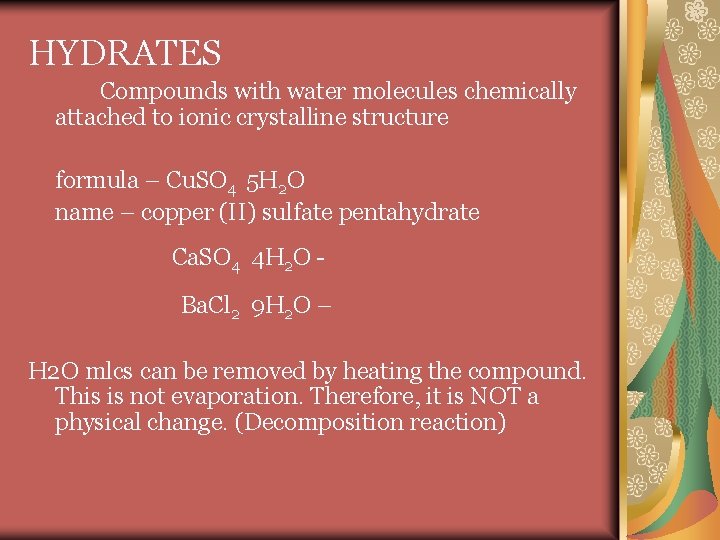
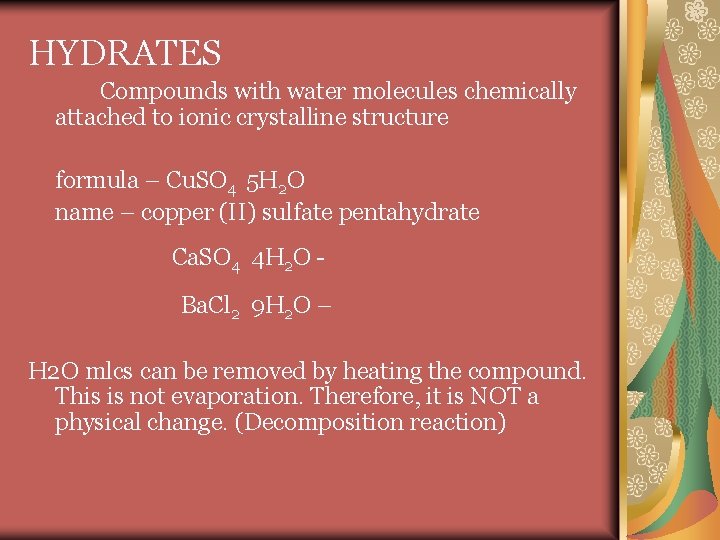
HYDRATES Compounds with water molecules chemically attached to ionic crystalline structure formula – Cu. SO 4 5 H 2 O name – copper (II) sulfate pentahydrate Ca. SO 4 4 H 2 O Ba. Cl 2 9 H 2 O – H 2 O mlcs can be removed by heating the compound. This is not evaporation. Therefore, it is NOT a physical change. (Decomposition reaction)

HYDRATES Compounds with water molecules chemically attached to ionic crystalline structure formula – Cu. SO 4 5 H 2 O name – copper (II) sulfate pentahydrate Ca. SO 4 4 H 2 O Ba. Cl 2 9 H 2 O – H 2 O mlcs can be removed by heating the compound. This is not evaporation. Therefore, it is NOT a physical change. (Decomposition reaction)

Mole-to-Mole Relationships Decomposition of water: 2 H 20 2 H 2 + O 2 2 mol of H 20 yields 2 mol H 2 and 1 mol O 2 Ex) You have 4 mol of water. If you decompose 4 mol of water, how many mols of products do you get? 2[2 H 2 O 2 H 2 + O 2] 4 H 2 O 4 H 2 + 2 O 2 4 mol of H 2 O yields 4 mol of H 2 plus 2 mol of O 2