New Insight in Hepatitis Profile Interpretation B Hepatitis

New Insight in Hepatitis Profile Interpretation B형간염의 이해
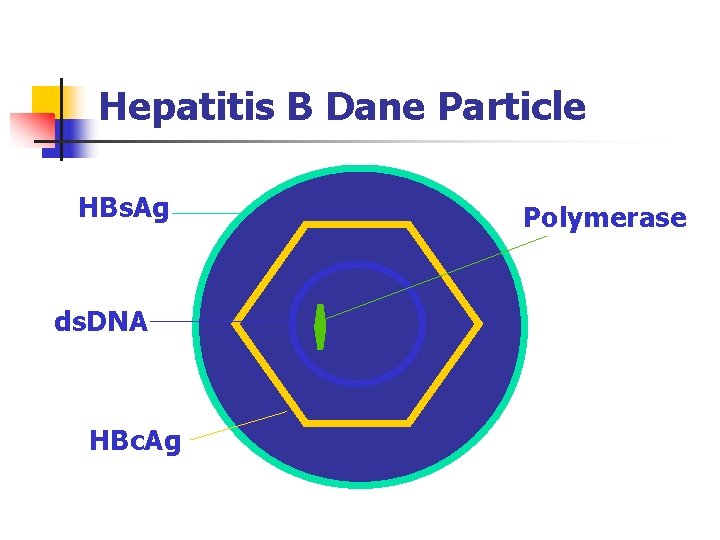
Hepatitis B Dane Particle HBs. Ag ds. DNA HBc. Ag Polymerase

HBV Genome Core Gene Core nucleocapsid protein & HBe. Ag Surface Gene Pre-S 1, pre-S 2, & S protein X Gene X protein Polymerase Gene A large protein with functions critical for packing & DNA replication

Serological and clinical patterns of acute HBV infections Symptoms HBe. Ag Anti-HBe Titer Total anti-HBc HBs. Ag Ig. M anti-HBc Wk after exposure Anti-HBs






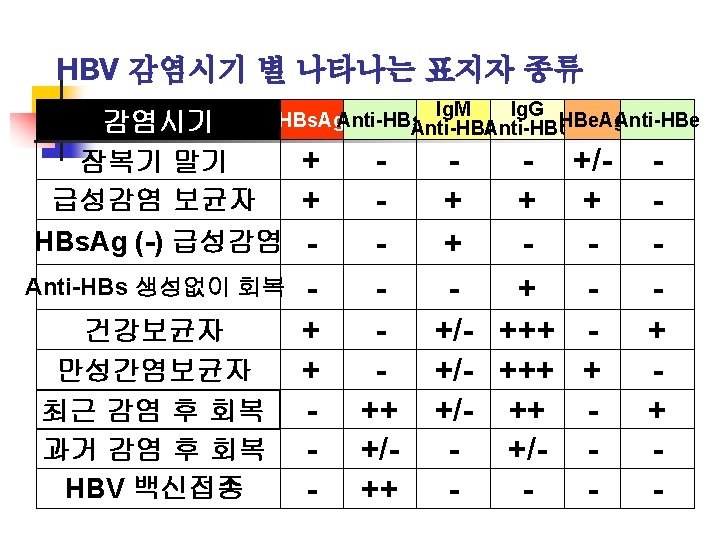


HBs. Ag은 양성인데 anti-HBc가 음성인 경우 1. HBs. Ag carrier infants 2. During the course of chronic hepatitis B virus infection 3. Blood donors positive for HBs. Ag and negative for anti-HBc antibody

HBs. Ag carrier infants HBs. Ag HBe. Ag Anti-HBc Anti-HBs Anti-HBe HBV DNA R • • NR NR P Occurred almost exclusively in children who were infected perinatally Immune incompetence to the HBV antigens Persist for more than 10 years Hepatitis B immunization did not have significant effect on the clinical course in carriers.
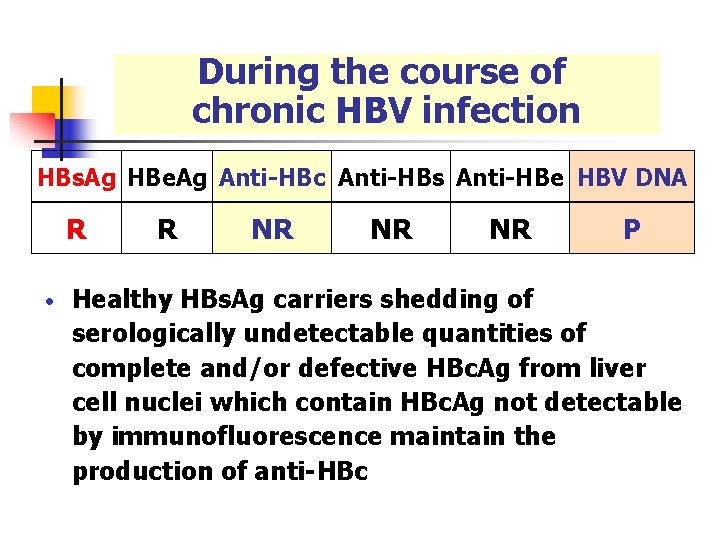
During the course of chronic HBV infection HBs. Ag HBe. Ag Anti-HBc Anti-HBs Anti-HBe HBV DNA R • R NR NR NR P Healthy HBs. Ag carriers shedding of serologically undetectable quantities of complete and/or defective HBc. Ag from liver cell nuclei which contain HBc. Ag not detectable by immunofluorescence maintain the production of anti-HBc
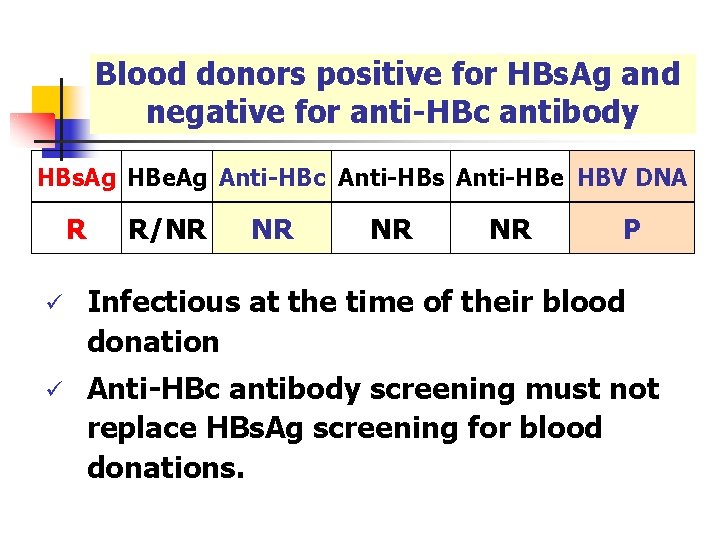
Blood donors positive for HBs. Ag and negative for anti-HBc antibody HBs. Ag HBe. Ag Anti-HBc Anti-HBs Anti-HBe HBV DNA R R/NR NR P ü Infectious at the time of their blood donation ü Anti-HBc antibody screening must not replace HBs. Ag screening for blood donations.






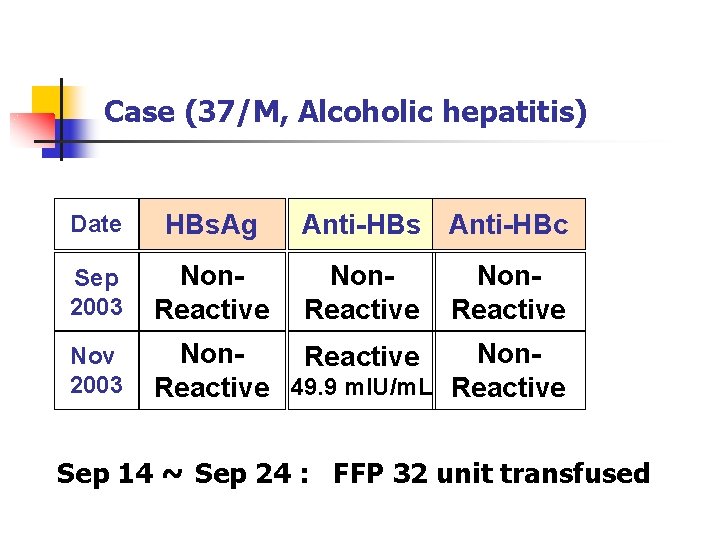
Case (37/M, Alcoholic hepatitis) Date HBs. Ag Anti-HBs Anti-HBc Sep 2003 Non. Reactive Nov 2003 Non. Reactive 49. 9 m. IU/m. L Reactive Non. Reactive Sep 14 ~ Sep 24 : FFP 32 unit transfused

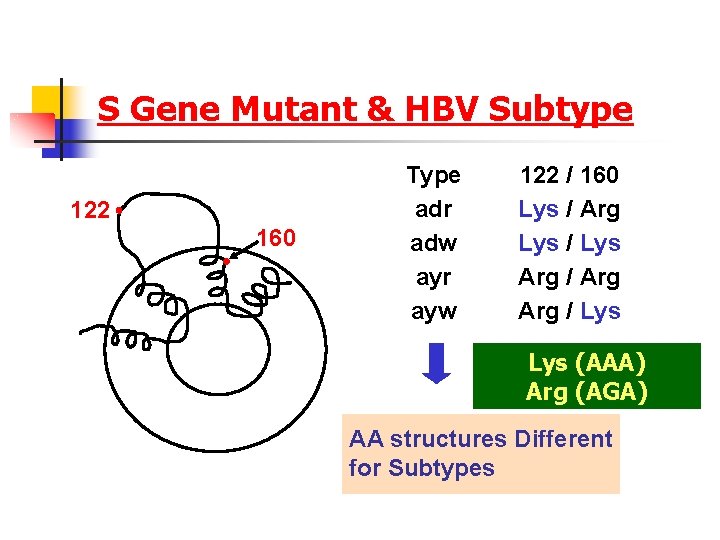
S Gene Mutant & HBV Subtype 122 160 Type adr adw ayr ayw 122 / 160 Lys / Arg Lys / Lys Arg / Lys (AAA) Arg (AGA) AA structures Different for Subtypes


Immunoprophylaxis in Liver Transplant Recipients B형간염과 관련된 질환으로 인하여 간이식술을 시행 받은 후 예방 목적으로 고농도의 HBIG을 투여 받는 환자 ü Immunosuppression ü HBIG-driven immune selection ü Prevalence 8. 3% ~ 62. 5%

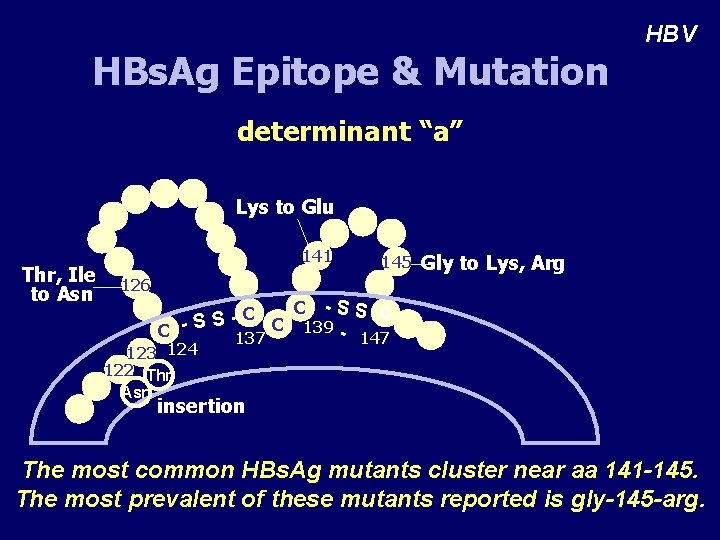
HBs. Ag Epitope & Mutation HBV determinant “a” Lys to Glu Thr, Ile to Asn 141 145 126 Gly to Lys, Arg C -SS C C S C 139 C -S 137 147 123 124 122 Thr Asn insertion The most common HBs. Ag mutants cluster near aa 141 -145. The most prevalent of these mutants reported is gly-145 -arg.

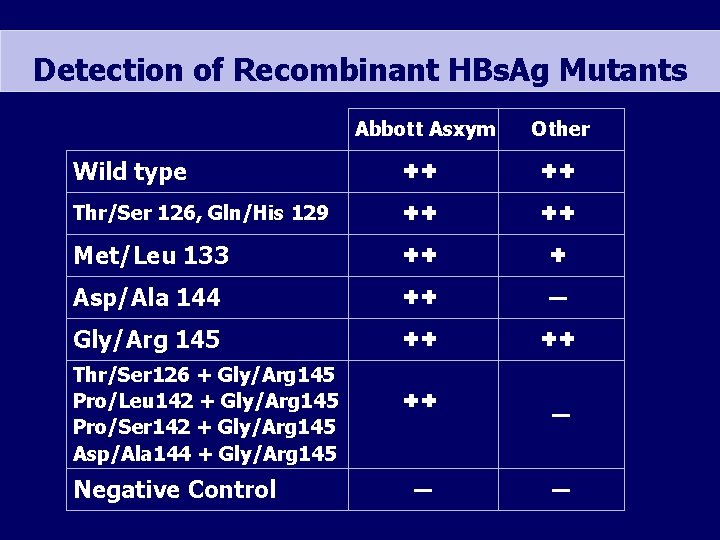
Detection of Recombinant HBs. Ag Mutants Abbott Asxym Other Wild type ✚✚ ✚✚ Thr/Ser 126, Gln/His 129 ✚✚ ✚✚ Met/Leu 133 ✚✚ ✚ Asp/Ala 144 ✚✚ ▬ Gly/Arg 145 ✚✚ ✚✚ Thr/Ser 126 + Gly/Arg 145 Pro/Leu 142 + Gly/Arg 145 Pro/Ser 142 + Gly/Arg 145 Asp/Ala 144 + Gly/Arg 145 Negative Control ✚✚ ▬ ▬ ▬

Prevention Why are HBs. Ag Mutants important? Vaccination Failure 1. 백신 접종을 실시하였지만 mutant HBV가 검 출될 수 있다. 2. HBV 만연지역에서는 HBs. Ag 양성 산모에서 태어나 HBIG 및 HBV 백신을 접종한 신생아의 2 -3%에서 vaccine escape mutant가 발견된 다.
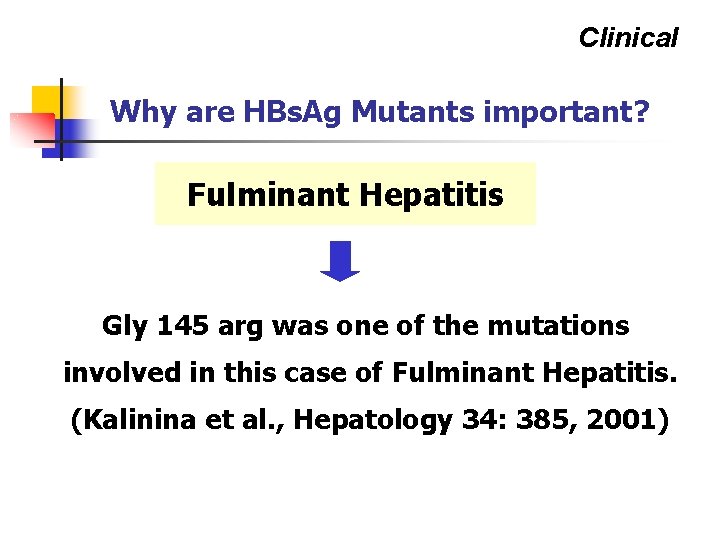
Clinical Why are HBs. Ag Mutants important? Fulminant Hepatitis Gly 145 arg was one of the mutations involved in this case of Fulminant Hepatitis. (Kalinina et al. , Hepatology 34: 385, 2001)


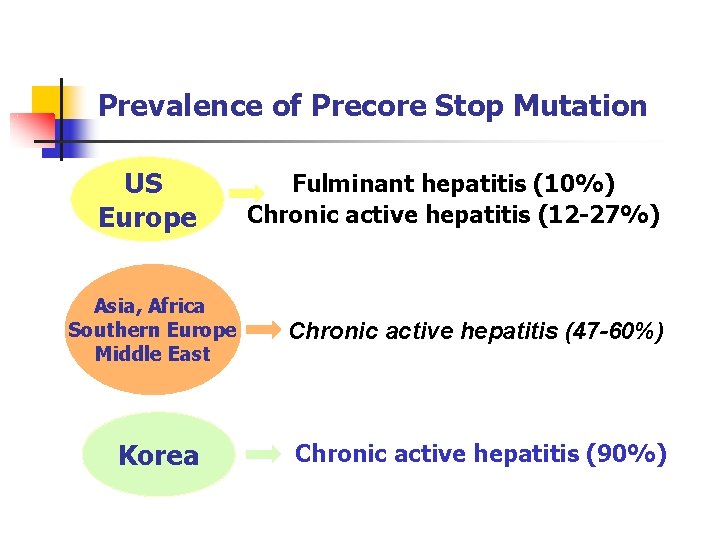
Prevalence of Precore Stop Mutation US Europe Fulminant hepatitis (10%) Chronic active hepatitis (12 -27%) Asia, Africa Southern Europe Middle East Chronic active hepatitis (47 -60%) Korea Chronic active hepatitis (90%)

Where have Precore Mutants been found § Healthy Chronic HBV Carriers § Patients with Fulminant Hepatitis § Patients with Aggressive Chronic Hepatitis B


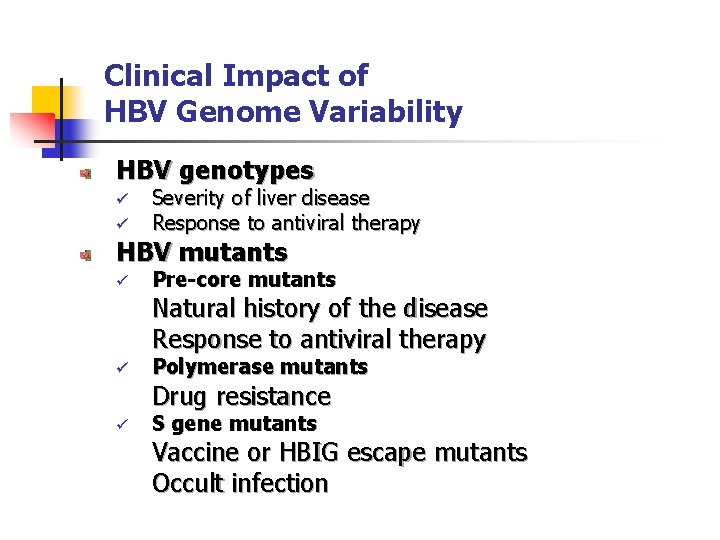
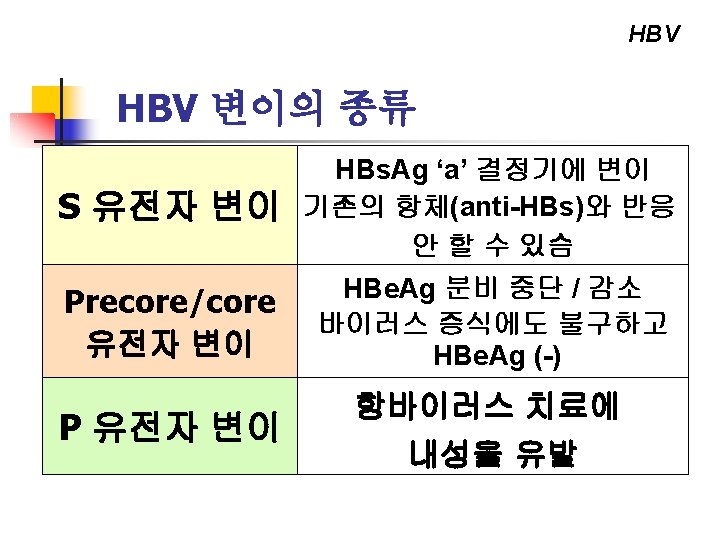
Clinical Impact of HBV Genome Variability HBV genotypes ü ü Severity of liver disease Response to antiviral therapy HBV mutants ü Pre-core mutants Natural history of the disease Response to antiviral therapy ü Polymerase mutants Drug resistance ü S gene mutants Vaccine or HBIG escape mutants Occult infection
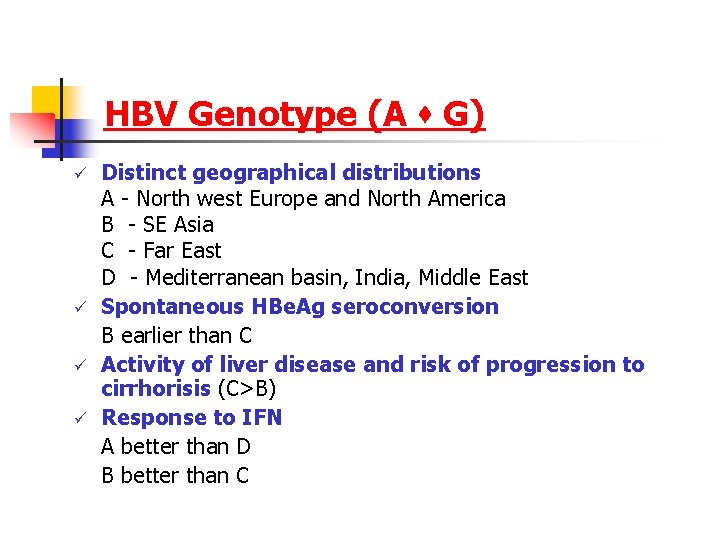
HBV Genotype (A s G) ü ü Distinct geographical distributions A - North west Europe and North America B - SE Asia C - Far East D - Mediterranean basin, India, Middle East Spontaneous HBe. Ag seroconversion B earlier than C Activity of liver disease and risk of progression to cirrhorisis (C>B) Response to IFN A better than D B better than C
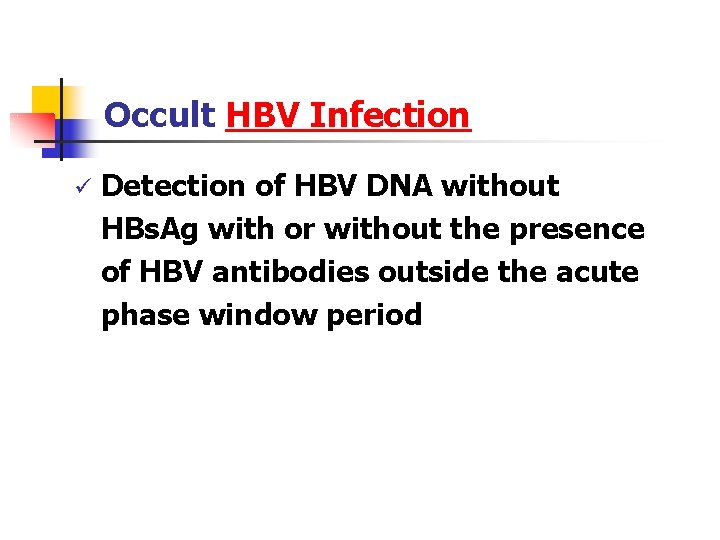
Occult HBV Infection ü Detection of HBV DNA without HBs. Ag with or without the presence of HBV antibodies outside the acute phase window period

Silent HBV Infection ü Presence of HBV DNA in serum or liver tissue in the absence of hepatitis B surface antigen (HBs. Ag), anti-HBc and anti-HBs in serum ü Caused in part by HBV mutants that produce a very low level of HBs. Ag, which escape detection by currently available serologic tests
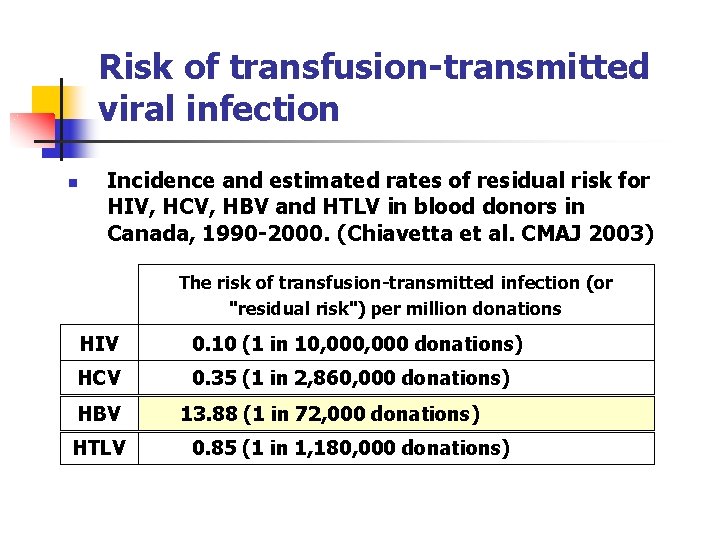
Risk of transfusion-transmitted viral infection n Incidence and estimated rates of residual risk for HIV, HCV, HBV and HTLV in blood donors in Canada, 1990 -2000. (Chiavetta et al. CMAJ 2003) The risk of transfusion-transmitted infection (or "residual risk") per million donations HIV 0. 10 (1 in 10, 000 donations) HCV 0. 35 (1 in 2, 860, 000 donations) HBV HTLV 13. 88 (1 in 72, 000 donations) 0. 85 (1 in 1, 180, 000 donations)
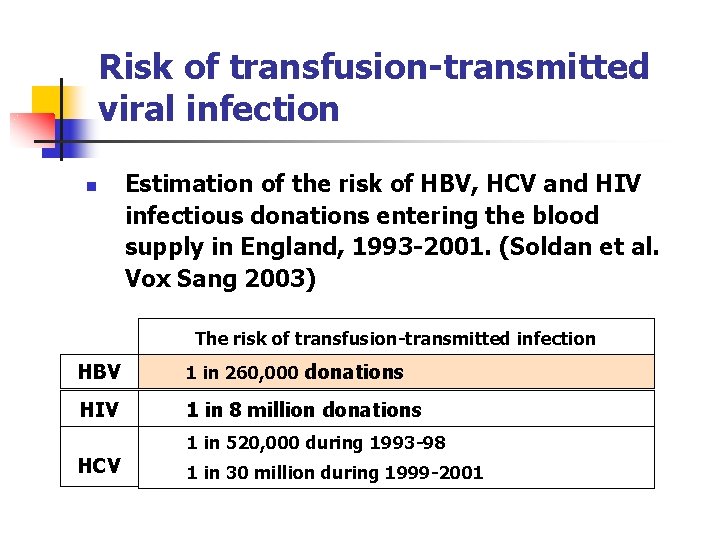
Risk of transfusion-transmitted viral infection n Estimation of the risk of HBV, HCV and HIV infectious donations entering the blood supply in England, 1993 -2001. (Soldan et al. Vox Sang 2003) The risk of transfusion-transmitted infection HBV 1 in 260, 000 donations HIV 1 in 8 million donations 1 in 520, 000 during 1993 -98 HCV 1 in 30 million during 1999 -2001

False-Negative Results In HBV Commercial Assays § HBs. Ag below the detection limit § Virus variants not recognized by the antibodies employed in the assays HBV
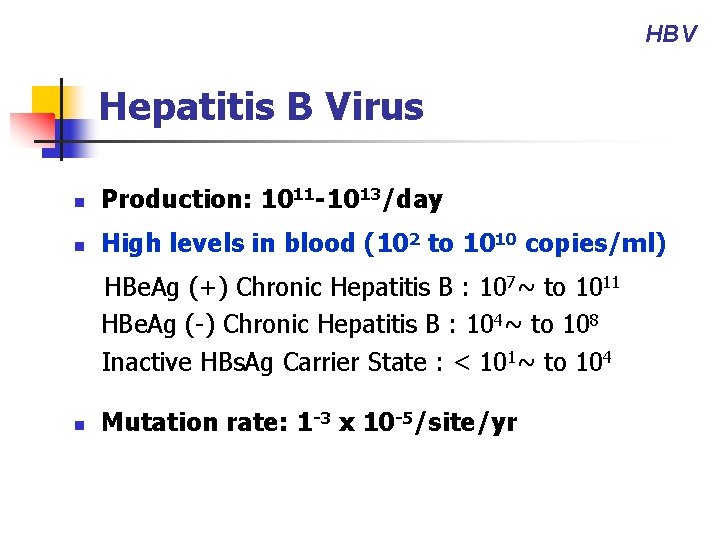
HBV Hepatitis B Virus n Production: 1011 -1013/day n High levels in blood (102 to 1010 copies/ml) HBe. Ag (+) Chronic Hepatitis B : 107~ to 1011 HBe. Ag (-) Chronic Hepatitis B : 104~ to 108 Inactive HBs. Ag Carrier State : < 101~ to 104 n Mutation rate: 1 -3 x 10 -5/site/yr

HBV HBs. Ag Below the Detection Limit n Window Period between Virus Exposure and the First Appearance of Detectable Antigen or Antibody n HBV Carriers Who Lack Detectable HBs. Ag
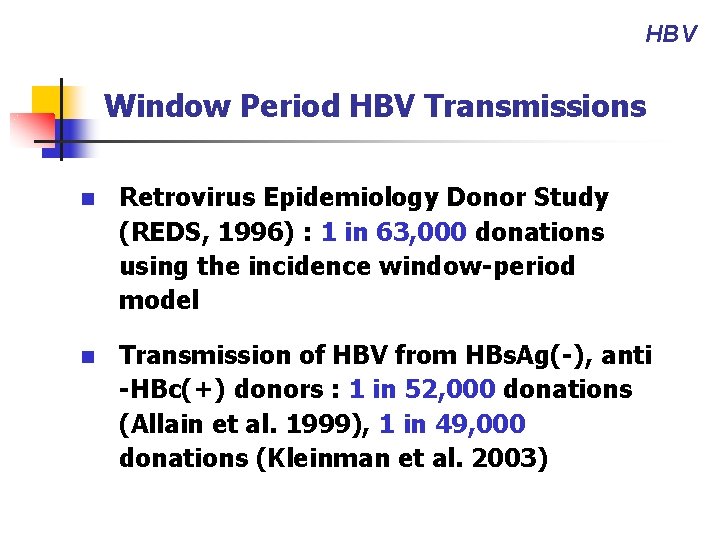
HBV Window Period HBV Transmissions n Retrovirus Epidemiology Donor Study (REDS, 1996) : 1 in 63, 000 donations using the incidence window-period model n Transmission of HBV from HBs. Ag(-), anti -HBc(+) donors : 1 in 52, 000 donations (Allain et al. 1999), 1 in 49, 000 donations (Kleinman et al. 2003)
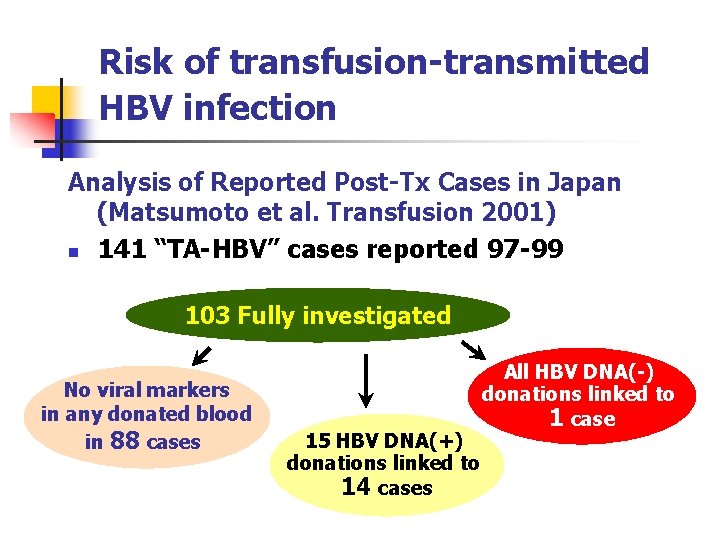
Risk of transfusion-transmitted HBV infection Analysis of Reported Post-Tx Cases in Japan (Matsumoto et al. Transfusion 2001) n 141 “TA-HBV” cases reported 97 -99 103 Fully investigated No viral markers in any donated blood in 88 cases 15 HBV DNA(+) donations linked to 14 cases All HBV DNA(-) donations linked to 1 case
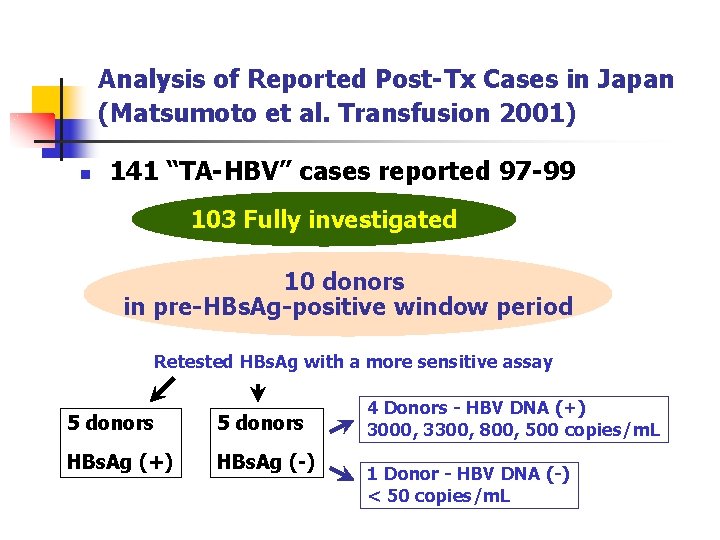
Analysis of Reported Post-Tx Cases in Japan (Matsumoto et al. Transfusion 2001) n 141 “TA-HBV” cases reported 97 -99 103 Fully investigated 10 donors in pre-HBs. Ag-positive window period Retested HBs. Ag with a more sensitive assay 5 donors HBs. Ag (+) HBs. Ag (-) 4 Donors - HBV DNA (+) 3000, 3300, 800, 500 copies/m. L 1 Donor - HBV DNA (-) < 50 copies/m. L
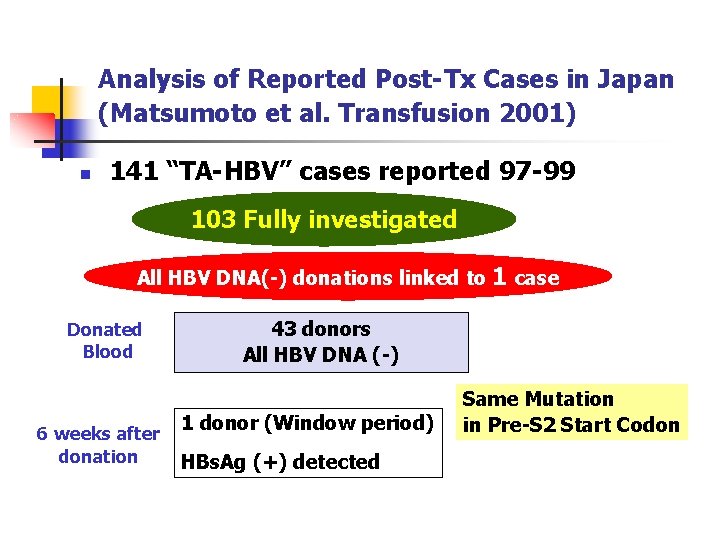
Analysis of Reported Post-Tx Cases in Japan (Matsumoto et al. Transfusion 2001) n 141 “TA-HBV” cases reported 97 -99 103 Fully investigated All HBV DNA(-) donations linked to Donated Blood 6 weeks after donation 1 case 43 donors All HBV DNA (-) 1 donor (Window period) HBs. Ag (+) detected Same Mutation in Pre-S 2 Start Codon
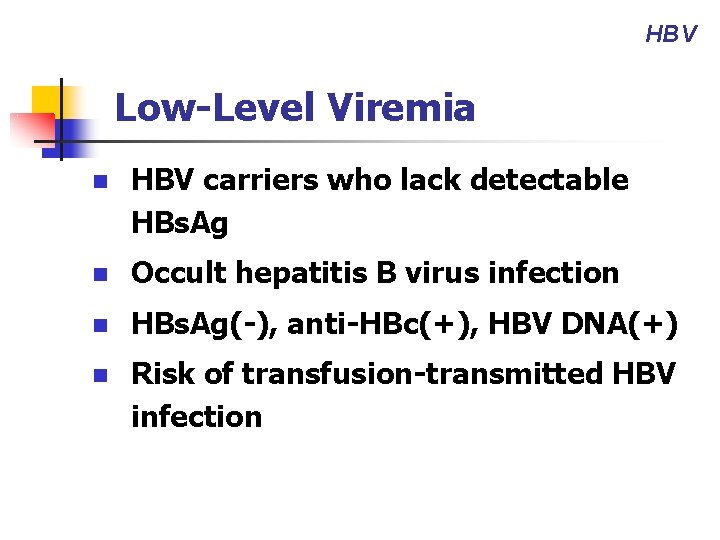
HBV Low-Level Viremia n HBV carriers who lack detectable HBs. Ag n Occult hepatitis B virus infection n HBs. Ag(-), anti-HBc(+), HBV DNA(+) n Risk of transfusion-transmitted HBV infection
HBV HBs. Ag(-), anti-HBc(+) n n HBV DNA is highly unlikely to be present in units with anti-HBs levels greater than 100 m. IU/m. L. Transfusion to anti-HBc-positive blood with high levels of anti-HBs (titer >1: 16, ≈ 200 m. IU/m. L) is permitted in Japan and no posttransfusion cases have been documented from such units.
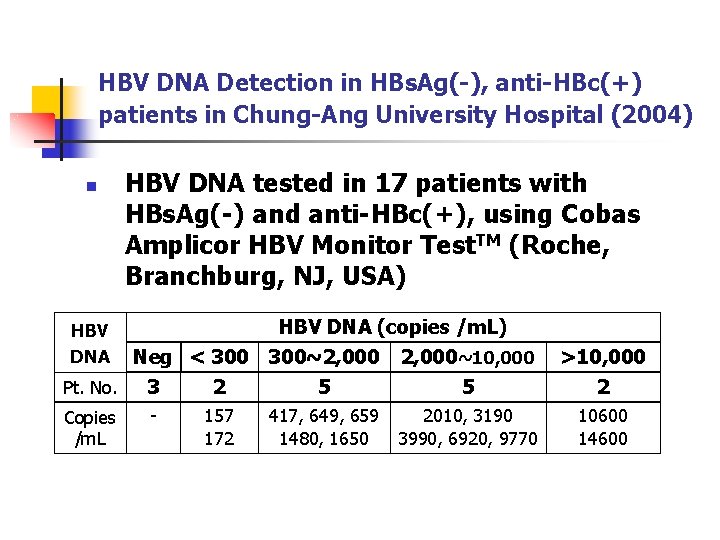
HBV DNA Detection in HBs. Ag(-), anti-HBc(+) patients in Chung-Ang University Hospital (2004) n HBV DNA tested in 17 patients with HBs. Ag(-) and anti-HBc(+), using Cobas Amplicor HBV Monitor Test. TM (Roche, Branchburg, NJ, USA) HBV DNA (copies /m. L) Neg < 300~2, 000~10, 000 Pt. No. 3 2 5 5 HBV DNA Copies /m. L - 157 172 417, 649, 659 1480, 1650 2010, 3190 3990, 6920, 9770 >10, 000 2 10600 14600
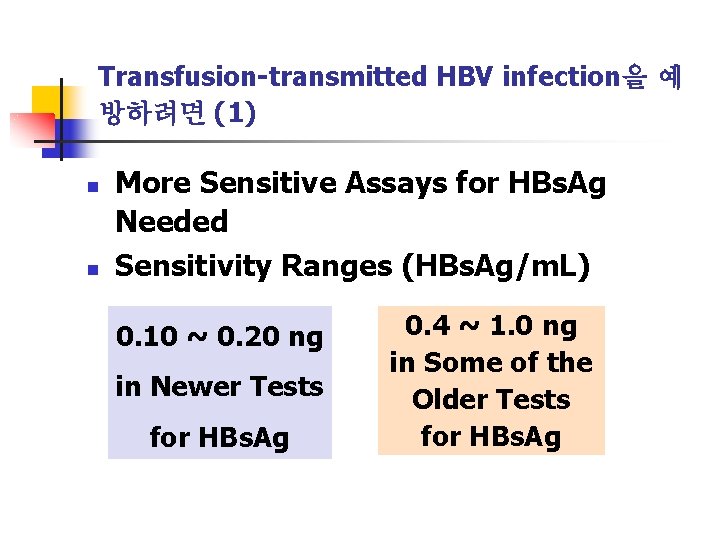
Transfusion-transmitted HBV infection을 예 방하려면 (1) n n More Sensitive Assays for HBs. Ag Needed Sensitivity Ranges (HBs. Ag/m. L) 0. 10 ~ 0. 20 ng in Newer Tests for HBs. Ag 0. 4 ~ 1. 0 ng in Some of the Older Tests for HBs. Ag
FDA RECOMMENDATIONS n FDA recommends that HBs. Ag detection assays that are used to test blood, blood components, and Source Plasma donations have a lower limit of detection capability of 0. 50 ng HBs. Ag/m. L or less.
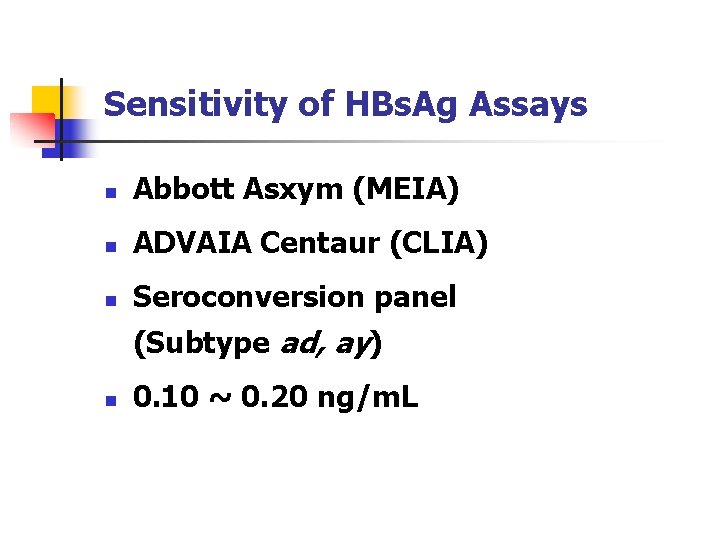
Sensitivity of HBs. Ag Assays n Abbott Asxym (MEIA) n ADVAIA Centaur (CLIA) n Seroconversion panel (Subtype ad, ay) n 0. 10 ~ 0. 20 ng/m. L

- Slides: 63