New Aspects of Adjuvant Therapy in Endometrial Cancer








- Slides: 20

New Aspects of Adjuvant Therapy in Endometrial Cancer: Current Standards and Future Directions Jalid Sehouli, Dominique Koensgen, Gulten Oskay-Ozcelik, Alexander Mustea Department of Obstetrics and Gynecology, Charite University Hospital, Campus Virchow-Clinic, Berlin, Germany Critical Reviews in Oncology/Hematology, February 2008

1. Introduction 2. Adjuvant therapy in early stage (stages I/II) 3. Stages III/IV and recurrent endometrial cancer 4. Future directions 5. Open questions 6. Conclusion

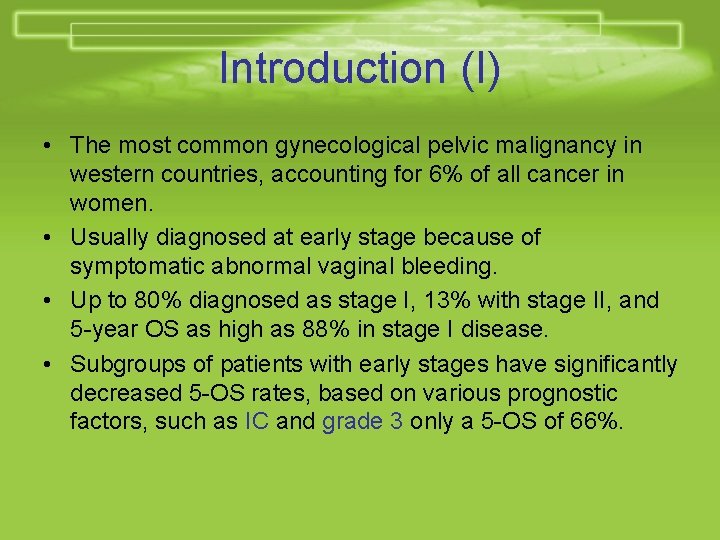
Introduction (I) • The most common gynecological pelvic malignancy in western countries, accounting for 6% of all cancer in women. • Usually diagnosed at early stage because of symptomatic abnormal vaginal bleeding. • Up to 80% diagnosed as stage I, 13% with stage II, and 5 -year OS as high as 88% in stage I disease. • Subgroups of patients with early stages have significantly decreased 5 -OS rates, based on various prognostic factors, such as IC and grade 3 only a 5 -OS of 66%.

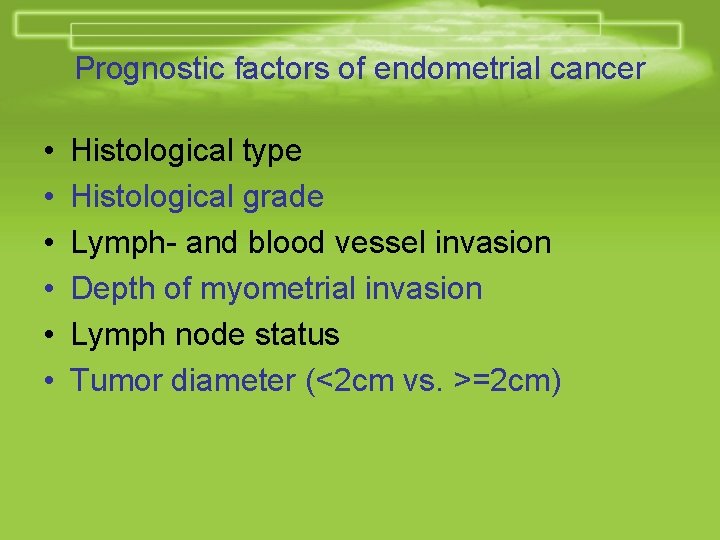
Prognostic factors of endometrial cancer • • • Histological type Histological grade Lymph- and blood vessel invasion Depth of myometrial invasion Lymph node status Tumor diameter (<2 cm vs. >=2 cm)

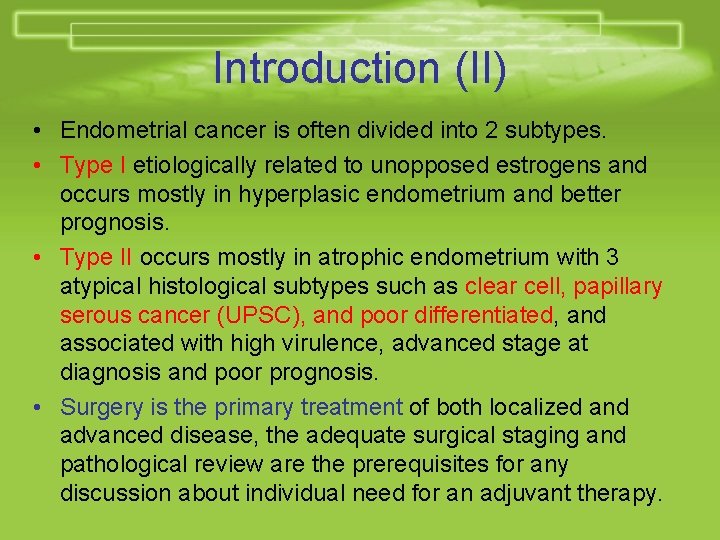
Introduction (II) • Endometrial cancer is often divided into 2 subtypes. • Type I etiologically related to unopposed estrogens and occurs mostly in hyperplasic endometrium and better prognosis. • Type II occurs mostly in atrophic endometrium with 3 atypical histological subtypes such as clear cell, papillary serous cancer (UPSC), and poor differentiated, and associated with high virulence, advanced stage at diagnosis and poor prognosis. • Surgery is the primary treatment of both localized and advanced disease, the adequate surgical staging and pathological review are the prerequisites for any discussion about individual need for an adjuvant therapy.

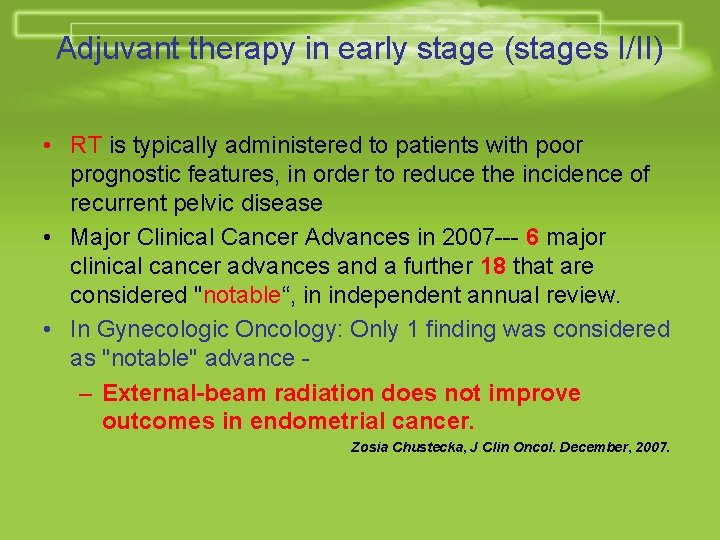
Adjuvant therapy in early stage (stages I/II) • RT is typically administered to patients with poor prognostic features, in order to reduce the incidence of recurrent pelvic disease • Major Clinical Cancer Advances in 2007 --- 6 major clinical cancer advances and a further 18 that are considered "notable“, in independent annual review. • In Gynecologic Oncology: Only 1 finding was considered as "notable" advance – External-beam radiation does not improve outcomes in endometrial cancer. Zosia Chustecka, J Clin Oncol. December, 2007.

Two important phase III randomized trials in 2007 A randomized phase III study on adjuvant treatment with radiation /- chemotherapy in early-stage high-risk endometrial cancer (NSGO-EC-9501/EORTC 55991). Hogberg T, Rosenberg P, Kristensen G, et al. Adjuvant external beam radiotherapy in the treatment of endometrial cancer: results of the randomized MRC ASTEC and NCIC CTG EN. 5 trial. Orton J, Blake P.

Results: NSGO-EC-9501/EORTC 55991 • • Median follow-up: 4. 3 years Statistically significant improvement in progression-free survival (hazard ratio 0. 62; P =. 03) in favor of the combined modality. Absolute difference in 5 -year progression-free survival: – 7% (79% vs. 72%). Cancer-specific overall survival at 5 years: – Improved in radiation/chemotherapy group (5% at 5 years). Combined modality improved progression-free but also cancer specific overall survival No difference of overall survival by randomization between combined modality and radiation alone Support for a role for adjuvant chemotherapy Hogberg T, et al. NSGO-EC-9501/EORTC 55991. ASCO 2007. Abstract 5503

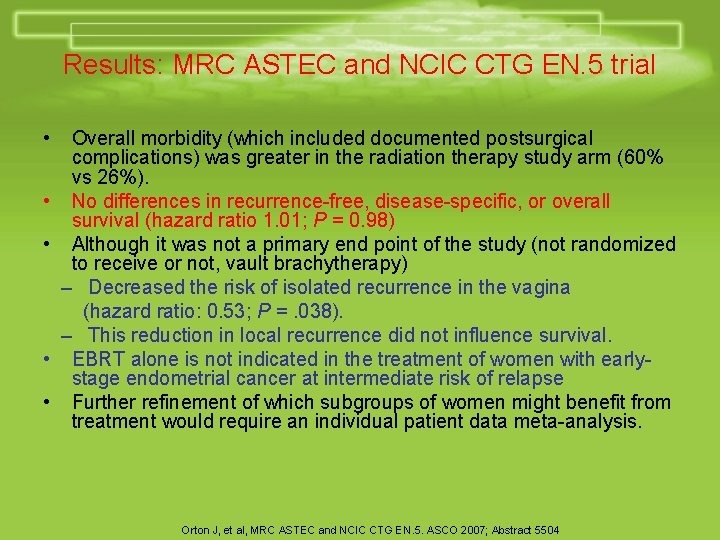
Results: MRC ASTEC and NCIC CTG EN. 5 trial • • • Overall morbidity (which included documented postsurgical complications) was greater in the radiation therapy study arm (60% vs 26%). No differences in recurrence-free, disease-specific, or overall survival (hazard ratio 1. 01; P = 0. 98) Although it was not a primary end point of the study (not randomized to receive or not, vault brachytherapy) – Decreased the risk of isolated recurrence in the vagina (hazard ratio: 0. 53; P =. 038). – This reduction in local recurrence did not influence survival. EBRT alone is not indicated in the treatment of women with earlystage endometrial cancer at intermediate risk of relapse Further refinement of which subgroups of women might benefit from treatment would require an individual patient data meta-analysis. Orton J, et al, MRC ASTEC and NCIC CTG EN. 5. ASCO 2007; Abstract 5504

Stage III/IV and recurrent endometrial cancer (I) • Clinicians recognize that patients with primary advanced and recurrent disease are in fact different patient populations, because of their heterogeneities in symptoms, tumor burden, tumor biology and prior treatment (e. g. radiation, chemotherapy). • Despite the good surgical outcome of patients with advanced endometrial cancer, approximately 50% of these patients will experience a recurrence of their cancer and will then later die due this malignant disease. • These patients may benefit from adjuvant platinum-based chemotherapy utilized to decrease the risk of cancer recurrence.

Whole abdominal irradiation vs. doxorubicin/cisplatin(60/50) regimen for stage III/IV endometiral cancer (GOG #122) Clinical data WAI CT No. of patients 202 194 No. of patients alive 38% 51% 4 8 Deaths from cancer 100 78 60 -Month PFS (corrected for stage) 60 -Month survival (corrected for stage) 38% 60% 42% 55% Treatment-related death

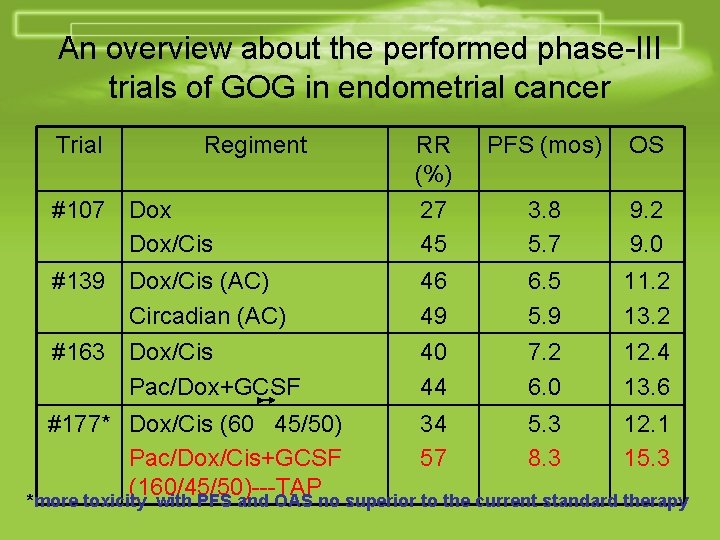
An overview about the performed phase-III trials of GOG in endometrial cancer Trial Regiment RR (%) PFS (mos) OS #107 Dox/Cis 27 45 3. 8 5. 7 9. 2 9. 0 #139 Dox/Cis (AC) Circadian (AC) 46 49 6. 5 5. 9 11. 2 13. 2 #163 Dox/Cis Pac/Dox+GCSF 40 44 7. 2 6. 0 12. 4 13. 6 #177* Dox/Cis (60 45/50) 34 5. 3 12. 1 Pac/Dox/Cis+GCSF 57 8. 3 15. 3 (160/45/50)---TAP *more toxicity with PFS and OAS no superior to the current standard therapy

Stage III/IV and recurrent endometrial cancer (II) • Doxorubicin is one of the most active agents in endometrial cancer and has formed the backbone of modern combination regimens. • The combination of CDDP+DOX accepted as the standard regimen for advanced or relapsed endometrial cancer. • Pegylated liposomal doxorubicin demonstrated the feasibility and activity of combination with carboplatin in advanced endometiral cancer, and well tolerated and offers promising activity in prospective randomized study. • GOG #209 had launched a large phase-III trial, compare carboplatin/paclitaxel to TAP in women with stage III/IV, or recurrent endometrial cancer.

Future directions (I) • Despite the availability of conventional factor such as ER/PR status and tumor histology, there are currently no validated molecular markers which have been identified in order to make any treatment decisions. • A loss of tumor suppressor gene PTEN is seen about 50% of endometrial cancer and PTEN mutations have been identified in endometrial hyperplasia, which targeting the rapamycin (m. TOR) pathway. • Three phase-II trials have been opened to explore the activity of m. TOR inhibitors (AP 23573, CCI-779, and RAD 001) in recurrent or metastastic endometrial cancer.

Future directions (II) • Newer targeting agents are being developed with the aim of achieving a higher specificity towards a specific population of tumor cells or toward any specific molecule of the cell cycle, such as epidermal growth factor receptors (EGFR) or vascular endothelial growth factor. • Similar to breast cancer, USPC can over express the HER 2/neu protein about 15%, the value of a therapy with trastuzumab (Herceptin) is unclear. • EGFR is over expressed in up to 80%, and associated with advanced stage and poor prognosis, erlotinib (Tarceva) and ZD 1839 (gefitinib) are TK inhibitors have been tested as a phase-II trial. • Currently, the GOG is assessing GW 572016 (lapatinib) in patients with advanced and recurrent endometrial cancer, and testing the combination of imatinib (Glivec) and paclitaxel in advanced and recurrent USPC.

Open questions (I) • For high-risk endometrial cancer patients: is a radiochemotherapy superior to chemotherapy alone? • For high-risk endometrial cancer in general: is chemotherapy superior to irradiation? • What is the role of radiochemotherapy or systemic chemotherapy in patients who received systemic lymph node dissection in case of negative or positive lymph node metastases?

Open questions (II) • How can we improve the toxicity of systemic chemotherapy without compromising the efficacy for patients with advanced stage? • Is there any place for new targeted agents as maintenance therapy in advanced endometrial cancer? • What is the best adjuvant therapy for UPSC?

Conclusion • Pivotal randomized phase-III studies have demonstrated the efficacy of combination chemotherapy for women with advanced or recurrent endometrial cancer. However, the long-term results remain unsatisfying. • Participation in clinical trials is the only way to achieve any progress in the clinical management of patients with endometrial cancer, and this may answer many questions the still exist. • Besides outcome parameters such as PFS and OAS, it is the Qo. L which should be defined as a primary objective in future clinical trials for patients with endometrial cancer.

Comments • The incidence of endometrial cancer has increased 235% from 1995 to 2005 in Taiwan, and it will be most common and important cancer of gynecological malignancies in the future. • The treatment of endometrial cancer has evolved and changed in the past decades, so our guideline of treatment must be deliberated and update. • We should participate in international global clinic trials or cooperate multi-center hospitals clinical trials on the opportunity, or design our protocols for management of endometrial cancer.

Thank you for your attention
 Endometrial cancer
Endometrial cancer Endometrial cancer
Endometrial cancer Gondor adjuvant
Gondor adjuvant Adjuvant nsclc
Adjuvant nsclc Adjuvant neoadjuvant palliative
Adjuvant neoadjuvant palliative Adjuvant nsclc
Adjuvant nsclc Cuerpo amarillo
Cuerpo amarillo Medidas del endometrio
Medidas del endometrio Spotting differential diagnosis
Spotting differential diagnosis Endometrial cells
Endometrial cells Projektov�� mana����r
Projektov�� mana����r Progesterone breakthrough bleeding
Progesterone breakthrough bleeding Uterine horn
Uterine horn Curritage
Curritage What is this organ
What is this organ Hiperplasia endometrial clasificación figo
Hiperplasia endometrial clasificación figo Endometrial histology menstrual cycle
Endometrial histology menstrual cycle Teratoma ovarium
Teratoma ovarium Proton therapy for breast cancer after mastectomy
Proton therapy for breast cancer after mastectomy Psychoanalytic vs humanistic
Psychoanalytic vs humanistic Bioness bits cost
Bioness bits cost