New AGA Devices PDA II Vascular Plug IV










- Slides: 45

New AGA Devices PDA II, Vascular Plug IV, Membranous VSD and Left Atrial Appendage Occluder Dr. John M. Lasala MD Ph. D Director Interventional Cardiology and Cardiac Cath Barnes-Jewish Hospital Professor of Medicine Washington University St. Louis, Missouri

Conflict of Interest Speaker and Proctor AGA Medical/ St Jude Medical Note: Some Products in this presentation are not FDA Approved

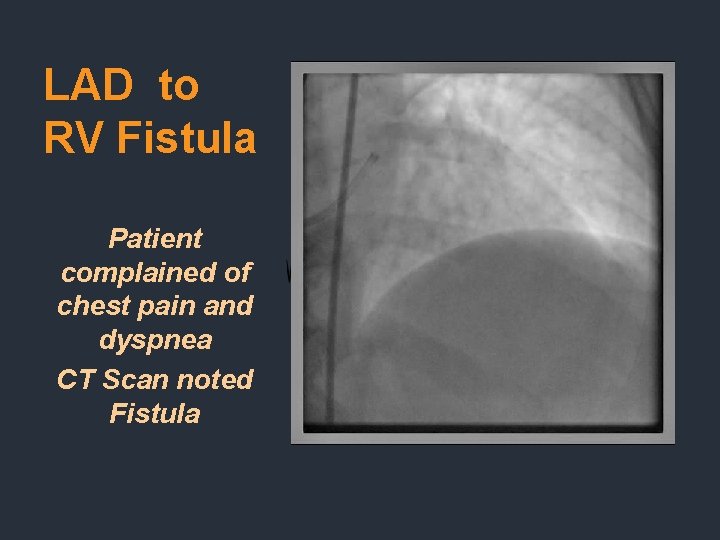
LAD to RV Fistula Patient complained of chest pain and dyspnea CT Scan noted Fistula

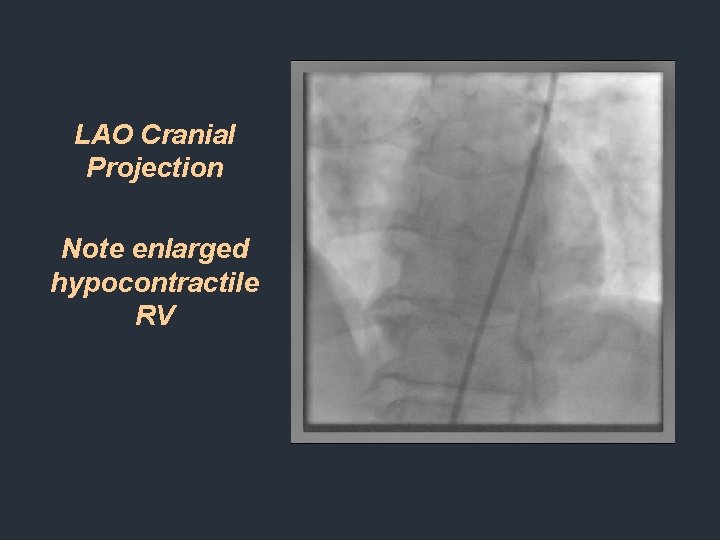
LAO Cranial Projection Note enlarged hypocontractile RV

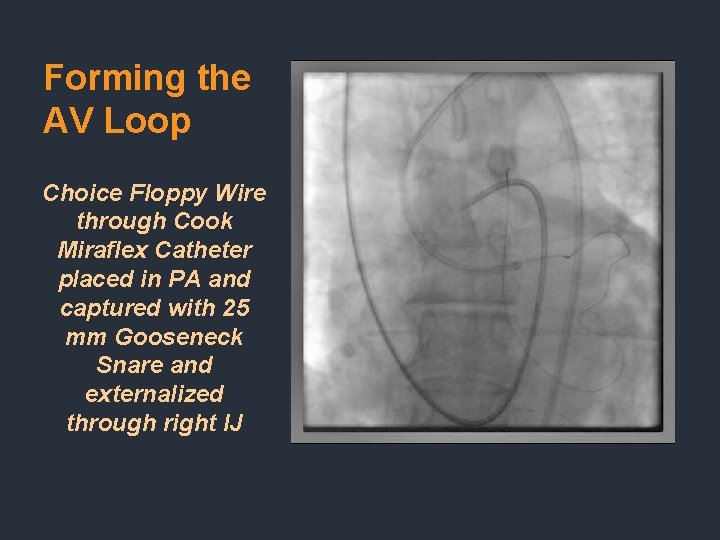
Forming the AV Loop Choice Floppy Wire through Cook Miraflex Catheter placed in PA and captured with 25 mm Gooseneck Snare and externalized through right IJ

Positionment of Delivery Catheter “Cut Down” 100 cm JR 4 Catheter or 55 cm Renal Catheter advanced over AV loop into LAD

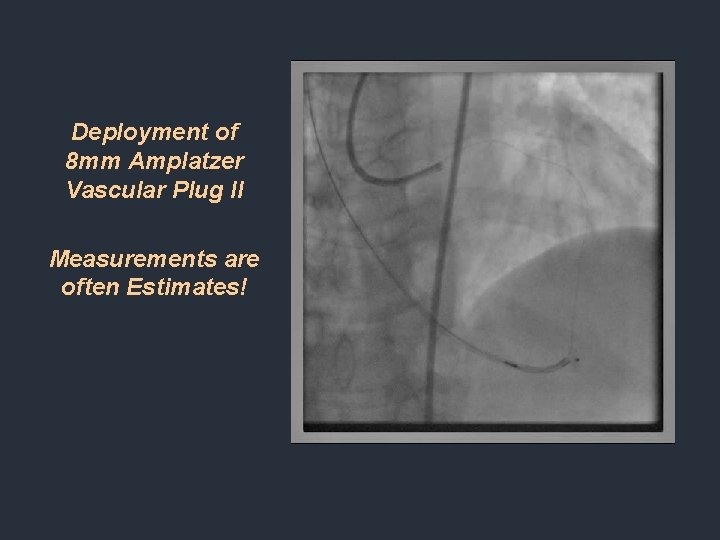
Deployment of 8 mm Amplatzer Vascular Plug II Measurements are often Estimates!

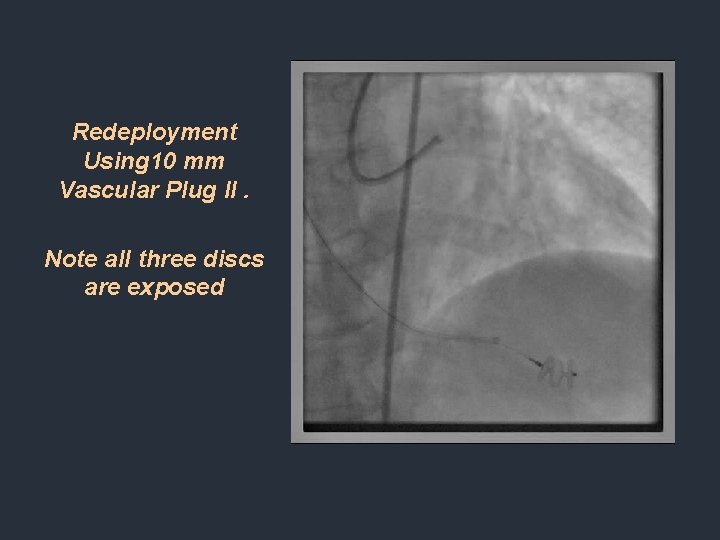
Redeployment Using 10 mm Vascular Plug II. Note all three discs are exposed

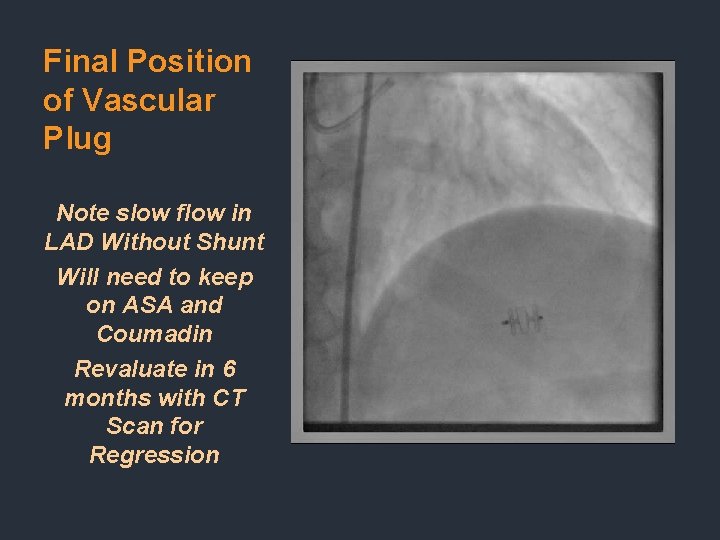
Final Position of Vascular Plug Note slow flow in LAD Without Shunt Will need to keep on ASA and Coumadin Revaluate in 6 months with CT Scan for Regression

Devices for Peripheral Vascular Occlusion AMPLATZER® Vascular Plug 4

Devices for Peripheral Vascular Occlusion AMPLATZER® Vascular Plug 4 Device description AGA Medical Corporation Dual-lobe, dual-layer Nitinol mesh with a floppy delivery wire tip for targeted delivery in distal and tortuous anatomy Delivery system 0. 035 Diagnostic Cath 4 Fr Device size 5 sizes from 4 mm to 8 mm Product Approval and Indication CE Mark Jul 2009 – Peripheral Vascular Occlusion

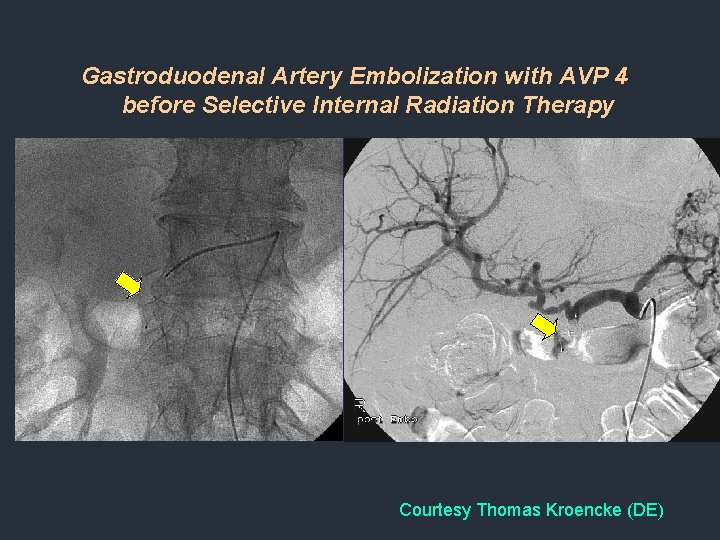
Gastroduodenal Artery Embolization with AVP 4 before Selective Internal Radiation Therapy Courtesy Thomas Kroencke (DE)

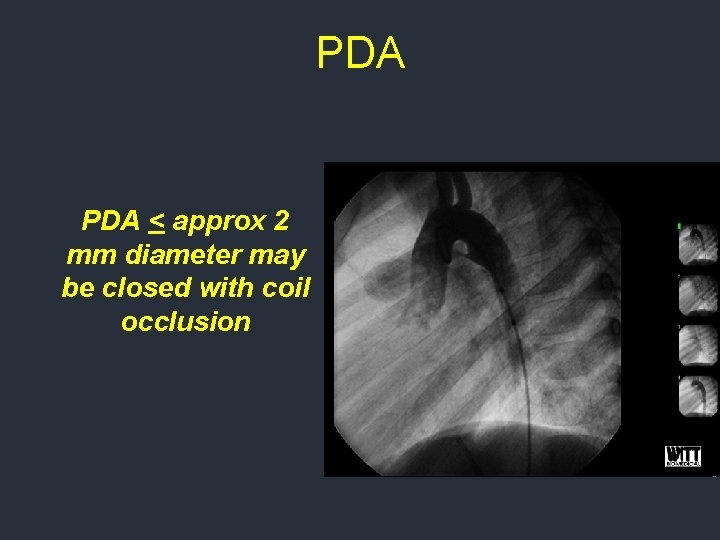
PDA Very common defect. True incidence unknown since many PDAs are silent Indications for closure include evidence of LV or LA enlargement or the presence of a heart murmur consistent with the PDA Treatment of “silent” PDAs controversial

PDA < approx 2 mm diameter may be closed with coil occlusion

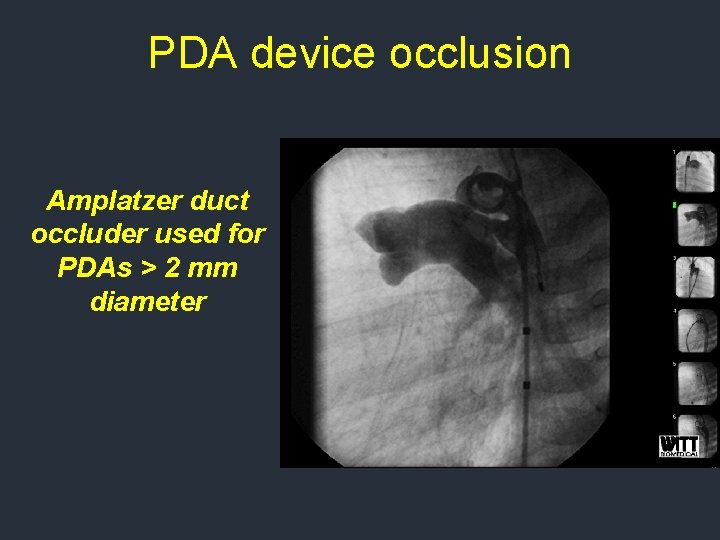
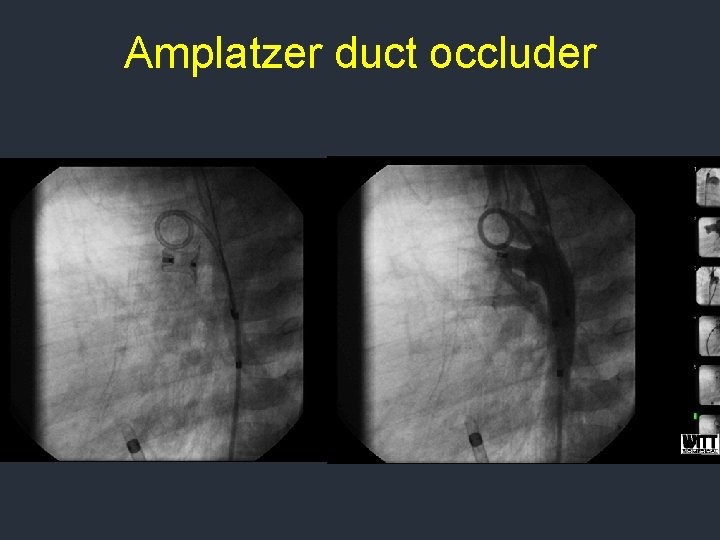
PDA device occlusion Amplatzer duct occluder used for PDAs > 2 mm diameter

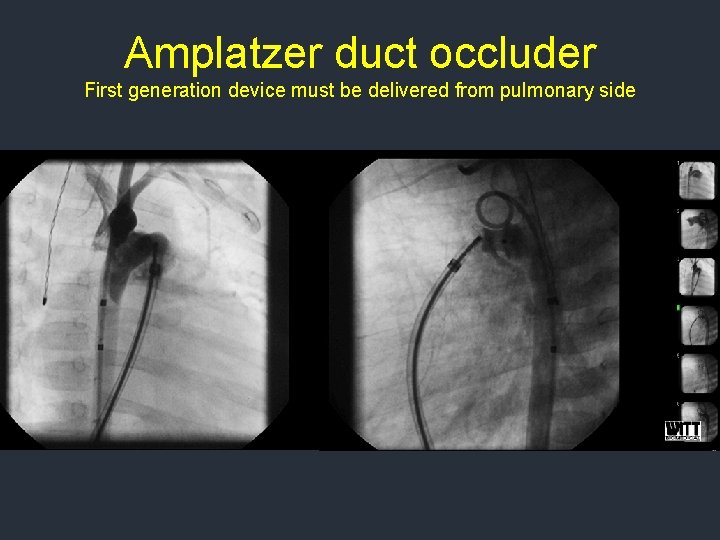
Amplatzer duct occluder First generation device must be delivered from pulmonary side

Amplatzer duct occluder

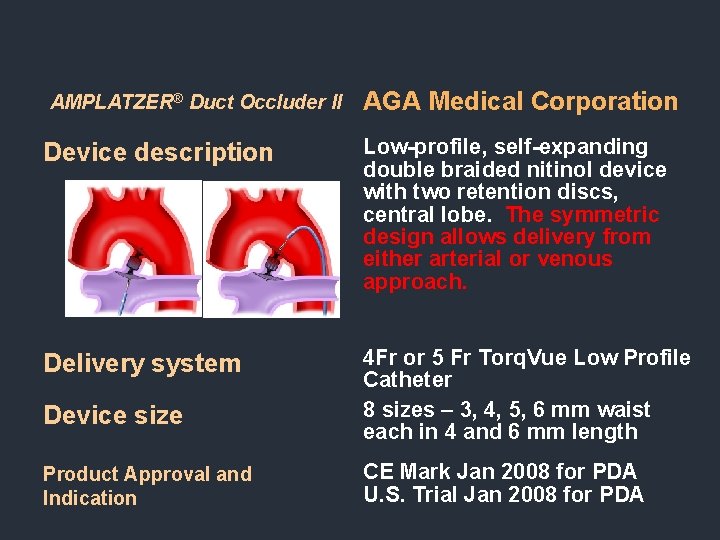
Devices for Closure of Structural Heart Defects AMPLATZER® Duct Occluder II

AMPLATZER® Duct Occluder II AGA Medical Corporation Device description Low-profile, self-expanding double braided nitinol device with two retention discs, central lobe. The symmetric design allows delivery from either arterial or venous approach. Delivery system 4 Fr or 5 Fr Torq. Vue Low Profile Catheter 8 sizes – 3, 4, 5, 6 mm waist each in 4 and 6 mm length Device size Product Approval and Indication CE Mark Jan 2008 for PDA U. S. Trial Jan 2008 for PDA

Devices for Closure of Structural Heart Defects AMPLATZER® Duct Occluder II Additional Sizes

AMPLATZER® Duct Occluder II Additional Sizes AGA Medical Corporation Device description Low-profile, self-expanding single-layer braided Nitinol device with two flat retention discs, central lobe, and micro end-screw designed to for less protrusion into the Aorta or Pulmonary Artery Delivery system 4 Fr Torq. Vue Low Profile Catheter 9 sizes – 3, 4, 5 mm waist each in 2, 4, 6 mm length Device size Product Approval and Indication CE Mark Jan 2011 – Patent Ductus Arteriosus

ADO II AS Implantation

Congenital VSDs are the most common form of congenital heart disease Approx 20% of pts with CHD have a VSD as the solitary lesion Echo studies demonstrate an incidence of 5 -50/1000 newborns Adult cardiologists mostly deal with post-MI VSDs

Congenital VSDs Ventricular septum viewed from the RV is divided into the inlet (I), outlet (O), and trabecular portions (T) Anatomic locations of VSDs include outlet (a), membranous (c), muscular(d, e, g), and inlet (f)

Congenital VSDs Inlet and outlet type VSDs are not currently amenable to transcatheter closure Muscular defects can be closed with either the Amplatzer muscular VSD occluder or the cardioseal device Membranous VSDs can be closed with the Amplatzer membranous VSD device but this is not currently approved in the US

VSD Types 1 – outflow portion of RV spontaneous closure uncommon 2 – perimembranous - most common – 80% 3 – inlet VSD in lower RV near TV, often in Down syndrome 4 – muscular, 20% of VSDs in infants, spontaneous closure is common Warnes CA, et. al. VSD. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease. J Am Coll Cardiol 2008; 52(23): e 173 -78.

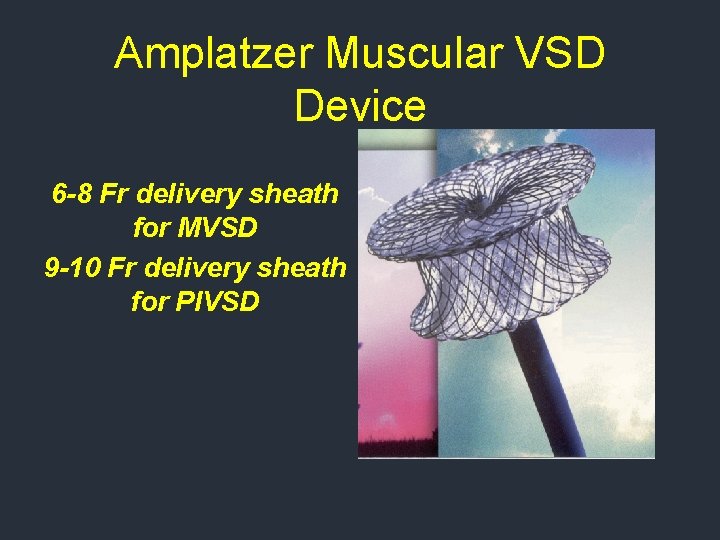
Amplatzer Muscular VSD Device 6 -8 Fr delivery sheath for MVSD 9 -10 Fr delivery sheath for PIVSD

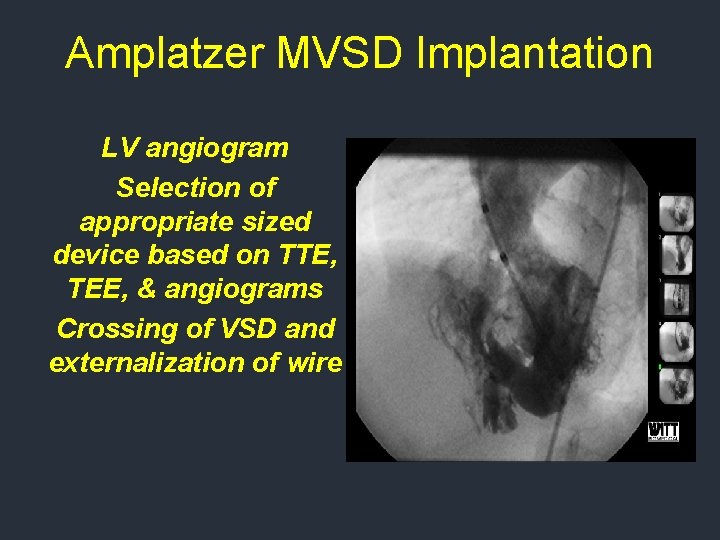
Amplatzer MVSD Implantation LV angiogram Selection of appropriate sized device based on TTE, TEE, & angiograms Crossing of VSD and externalization of wire

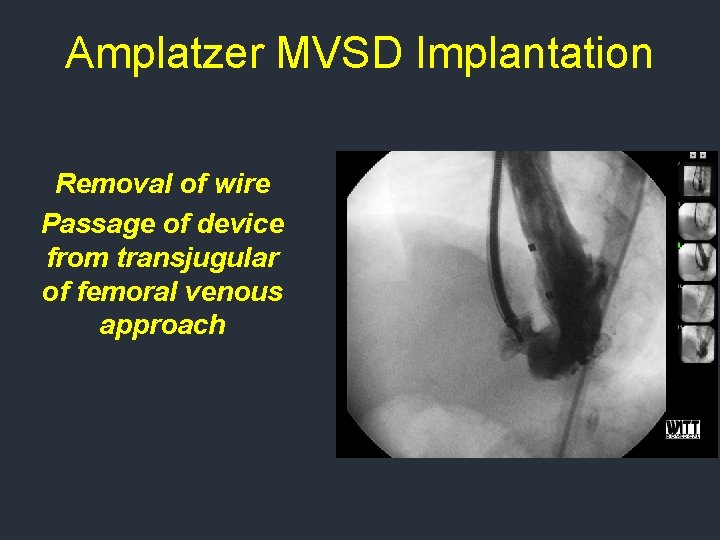
Amplatzer MVSD Implantation Removal of wire Passage of device from transjugular of femoral venous approach

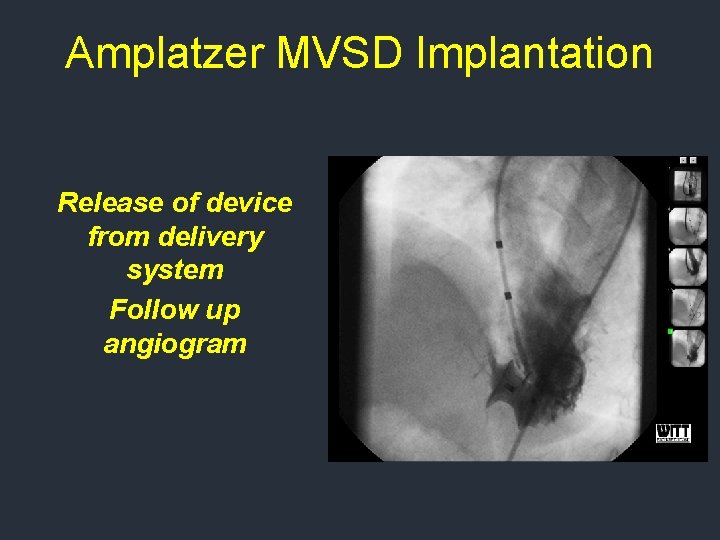
Amplatzer MVSD Implantation Release of device from delivery system Follow up angiogram

Devices for Closure of Structural Heart Defects AMPLATZER® Membranous VSD 2

AMPLATZER® Membranous VSD 2 AGA Medical Corporation Device description Dual-layer braided Nitinol device with central waist, LV sail/wingshaped disc, & RV circular disc designed to provided minimal force on the adjacent tissues while maintaining stability & closure Delivery system Device size Torq. Vue 4 Delivery System (6 -9 Fr) Pusher (6/7 & 8/9 Fr) 18 sizes 9 waist diameters: 4 -10, 12, 14 mm Each available in rim lengths: 1 mm & 3 mm Product Approval and Indication CE Mark Anticipated Q 2 2011 – Membranous VSD

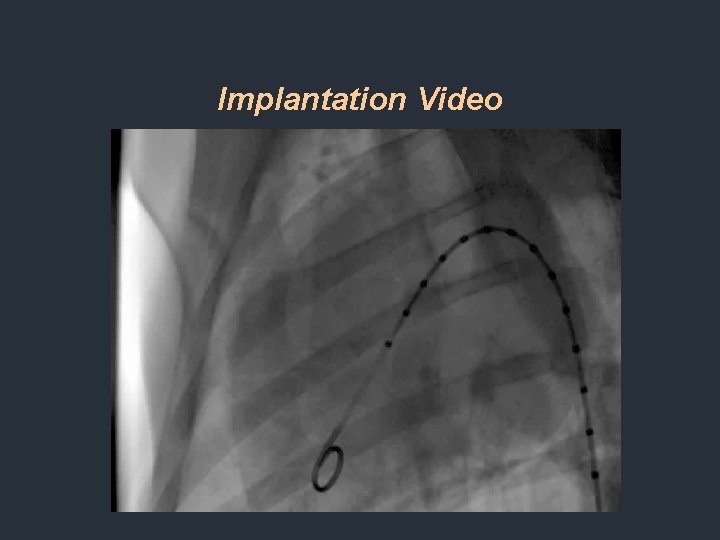
Implantation Video

Left Atrial Appendage Closure

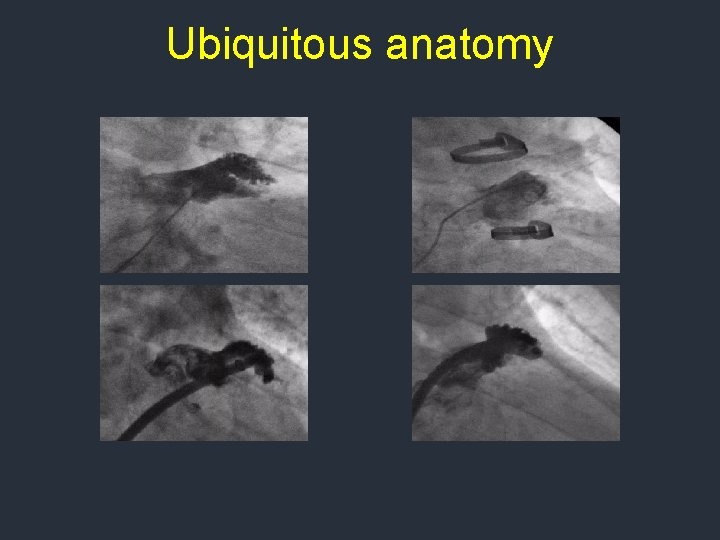
Ubiquitous anatomy

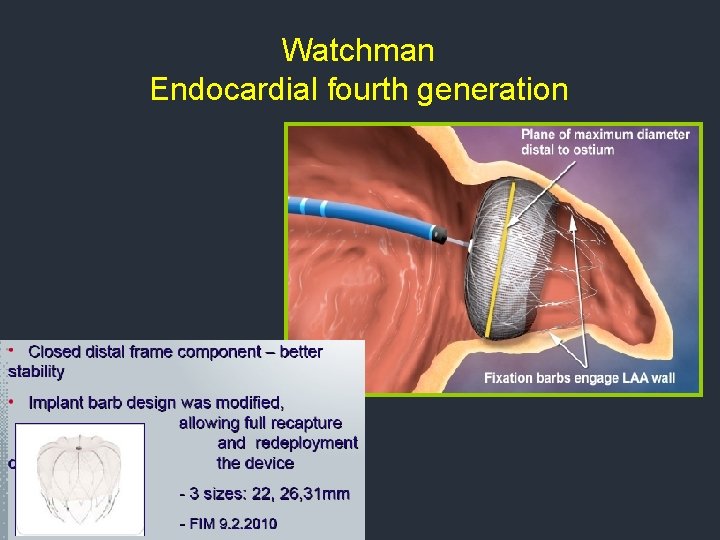
Watchman Endocardial fourth generation

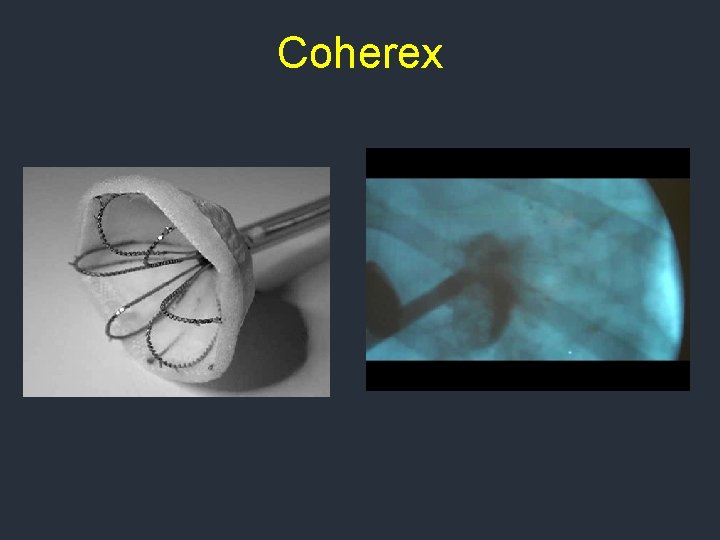
Coherex

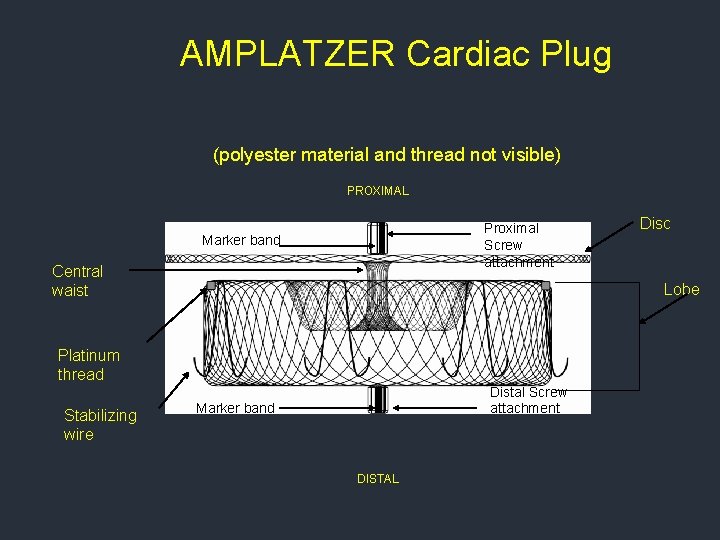
AMPLATZER Cardiac Plug (polyester material and thread not visible) PROXIMAL Proximal Screw attachment Marker band Central waist Lobe Platinum thread Stabilizing wire Disc Distal Screw attachment Marker band DISTAL

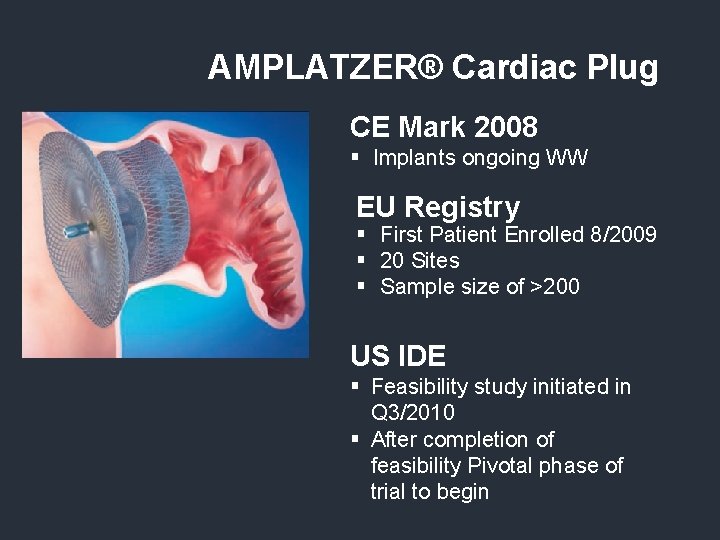
AMPLATZER® Cardiac Plug CE Mark 2008 § Implants ongoing WW EU Registry § First Patient Enrolled 8/2009 § 20 Sites § Sample size of >200 US IDE § Feasibility study initiated in Q 3/2010 § After completion of feasibility Pivotal phase of trial to begin

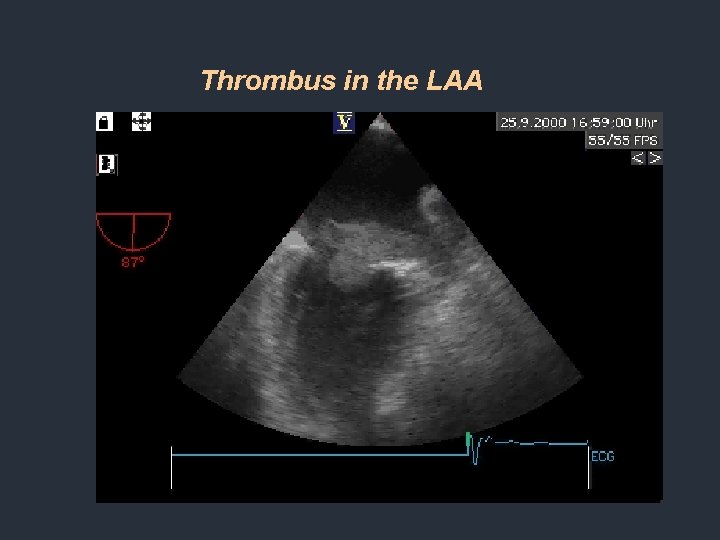
Thrombus in the LAA

AMPLATZER® Cardiac Plug

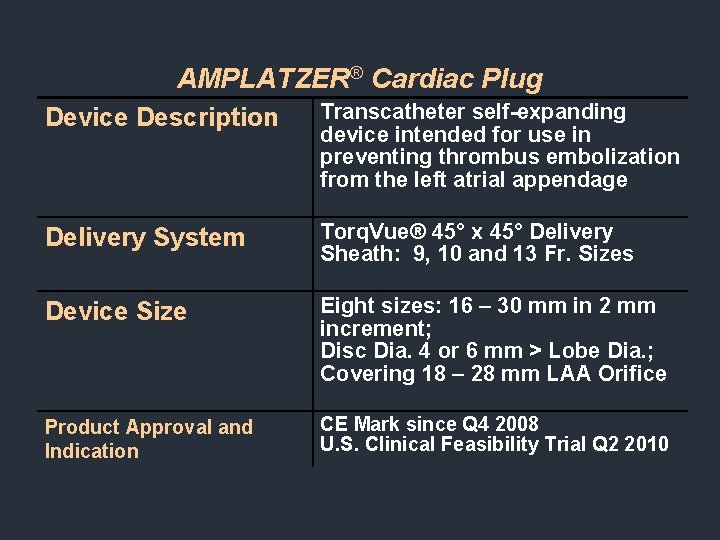
AMPLATZER® Cardiac Plug Device Description Transcatheter self-expanding device intended for use in preventing thrombus embolization from the left atrial appendage Delivery System Torq. Vue® 45° x 45° Delivery Sheath: 9, 10 and 13 Fr. Sizes Device Size Eight sizes: 16 – 30 mm in 2 mm increment; Disc Dia. 4 or 6 mm > Lobe Dia. ; Covering 18 – 28 mm LAA Orifice Product Approval and Indication CE Mark since Q 4 2008 U. S. Clinical Feasibility Trial Q 2 2010

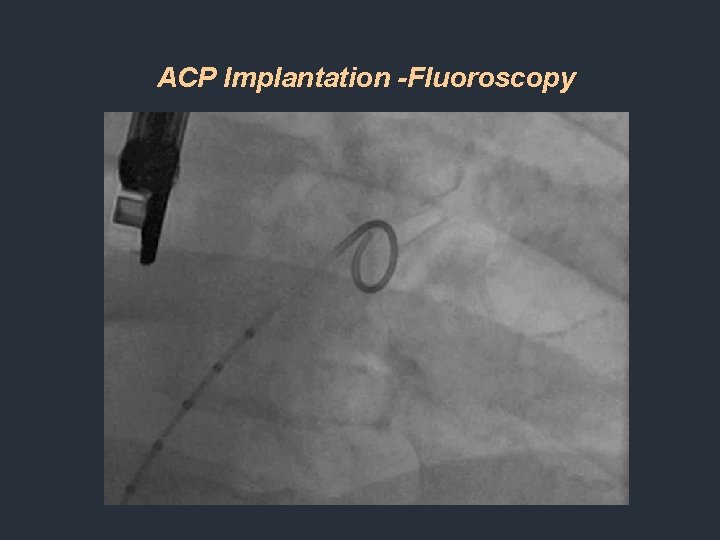
ACP Implantation -Fluoroscopy

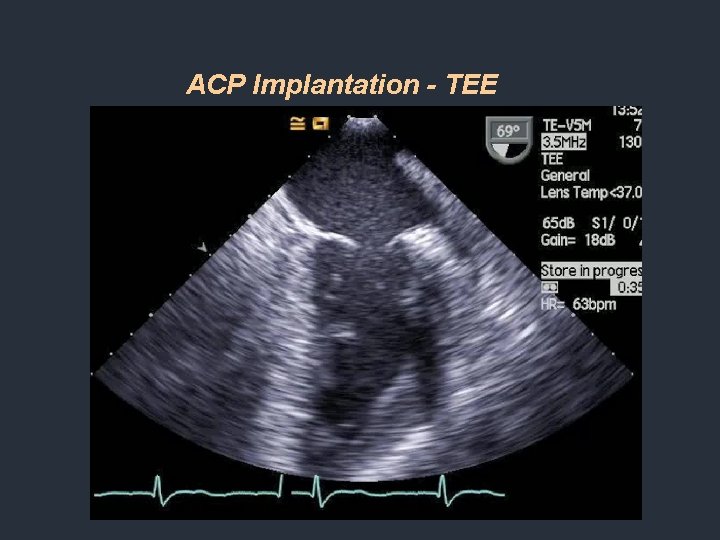
ACP Implantation - TEE

Conclusions 1. The newest Line of AGA products include upgrades of the vascular plug and ductal occluder and the introduction of the membranous VSD and cardiac LAA plug 2. None of these products are currently avavilable in the US but have ongoing European experience. 3. For now US physicians will have to make do with earlier generations. 4. The Cardiac LAA occluder hopes to begin randomized evaluation next year