Neutralization Reactions Acid Base Salt Water Double Replacement

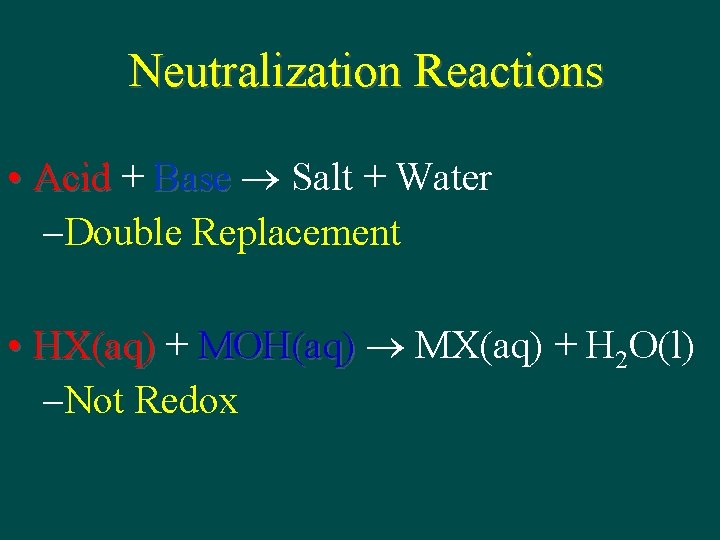
Neutralization Reactions • Acid + Base Salt + Water – Double Replacement • HX(aq) + MOH(aq) MX(aq) + H 2 O(l) – Not Redox
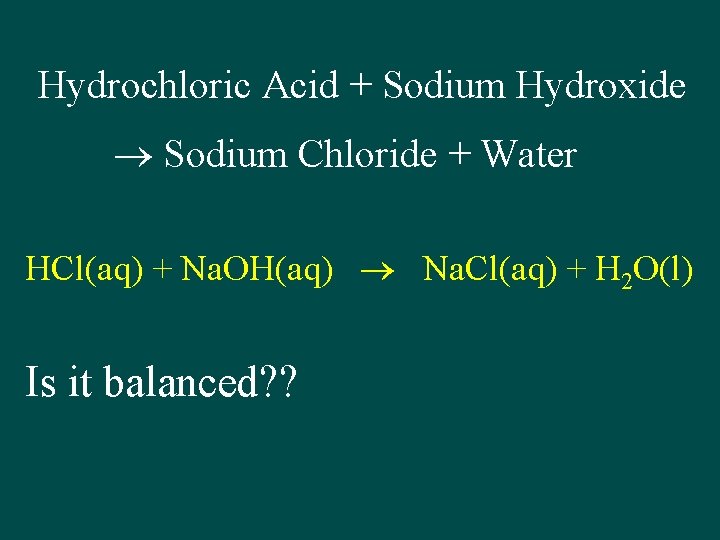
Hydrochloric Acid + Sodium Hydroxide Sodium Chloride + Water HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O(l) Is it balanced? ?
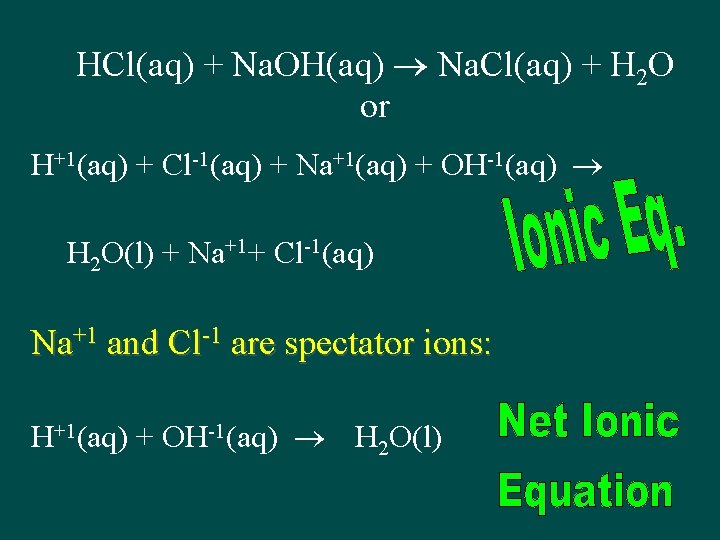
HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O or H+1(aq) + Cl-1(aq) + Na+1(aq) + OH-1(aq) H 2 O(l) + Na+1+ Cl-1(aq) Na+1 and Cl-1 are spectator ions: H+1(aq) + OH-1(aq) H 2 O(l)
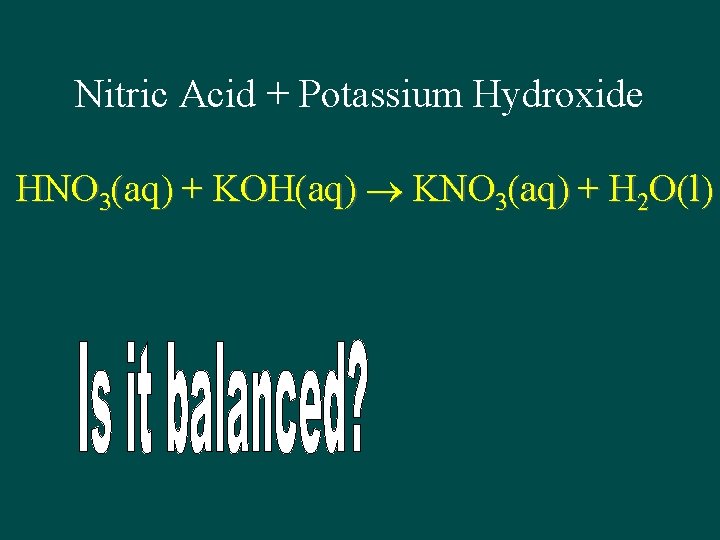
Nitric Acid + Potassium Hydroxide HNO 3(aq) + KOH(aq) KNO 3(aq) + H 2 O(l)
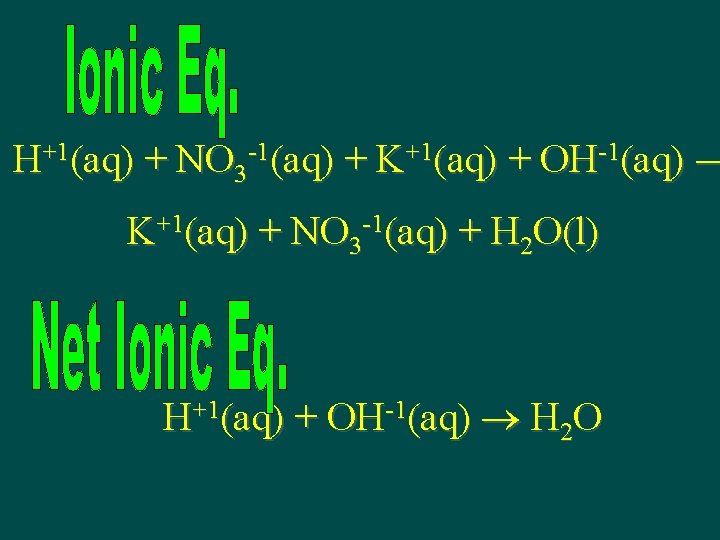
H+1(aq) + NO 3 -1(aq) + K+1(aq) + OH-1(aq) K+1(aq) + NO 3 -1(aq) + H 2 O(l) H+1(aq) + OH-1(aq) H 2 O
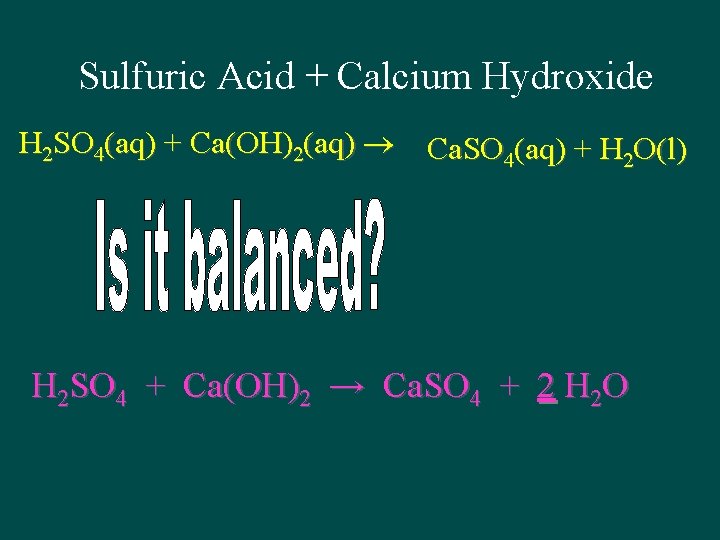
Sulfuric Acid + Calcium Hydroxide H 2 SO 4(aq) + Ca(OH)2(aq) Ca. SO 4(aq) + H 2 O(l) H 2 SO 4 + Ca(OH)2 → Ca. SO 4 + 2 H 2 O

For any neutralization reaction • The net ionic equation is always: +1 H + -1 OH H 2 O
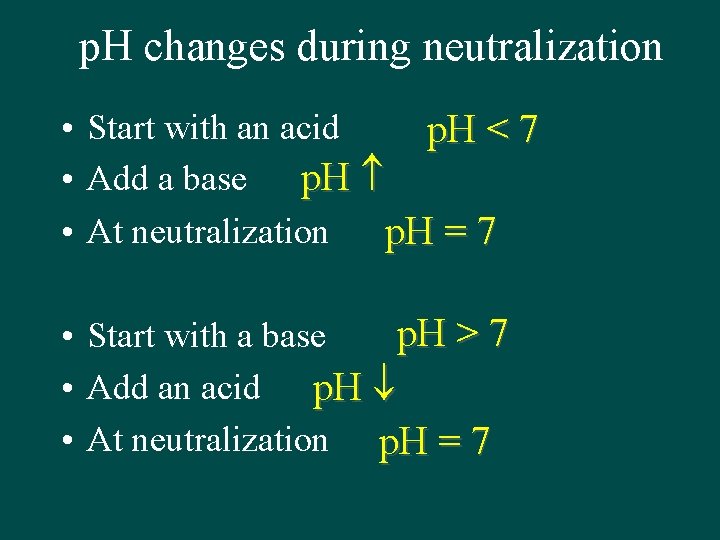
p. H changes during neutralization • Start with an acid p. H < 7 • Add a base p. H • At neutralization p. H = 7 p. H > 7 • Start with a base • Add an acid p. H • At neutralization p. H = 7
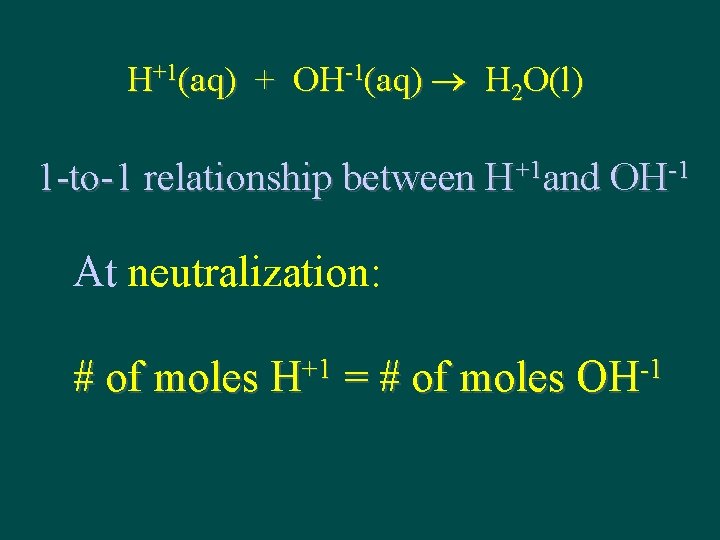
H+1(aq) + OH-1(aq) H 2 O(l) 1 -to-1 relationship between H+1 and OH-1 At neutralization: # of moles H+1 = # of moles OH-1
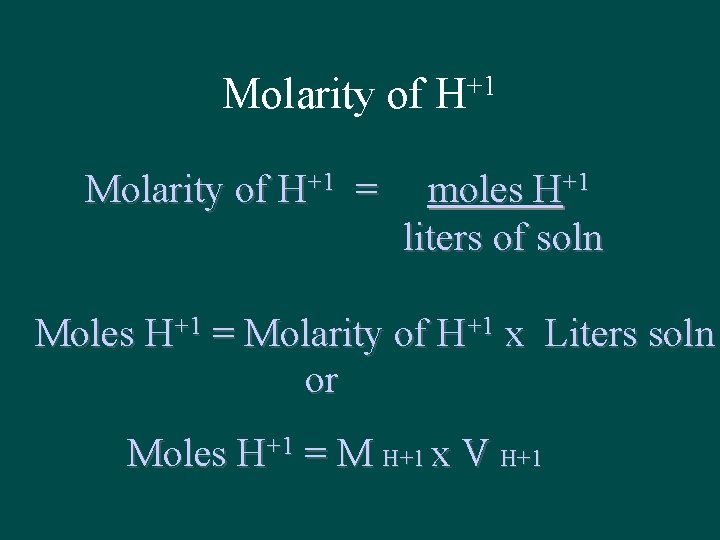
Molarity of H+1 = +1 H moles H+1 liters of soln Moles H+1 = Molarity of H+1 x Liters soln or Moles H+1 = M H+1 x V H+1
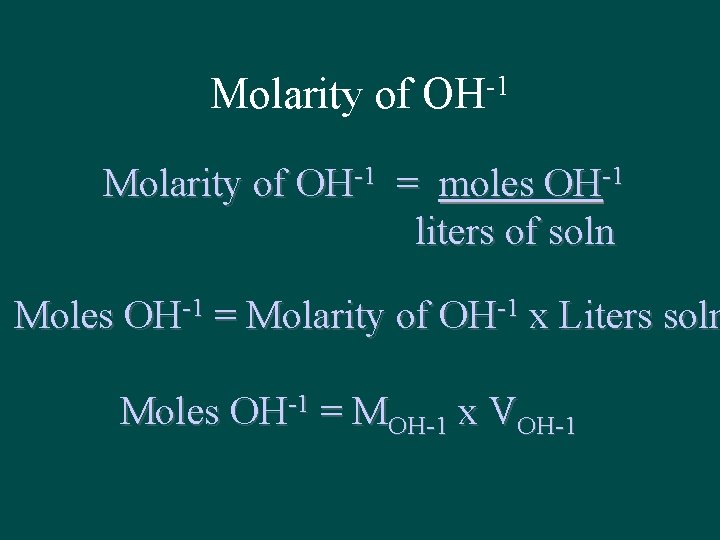
Molarity of -1 OH Molarity of OH-1 = moles OH-1 liters of soln Moles OH-1 = Molarity of OH-1 x Liters soln Moles OH-1 = MOH-1 x VOH-1
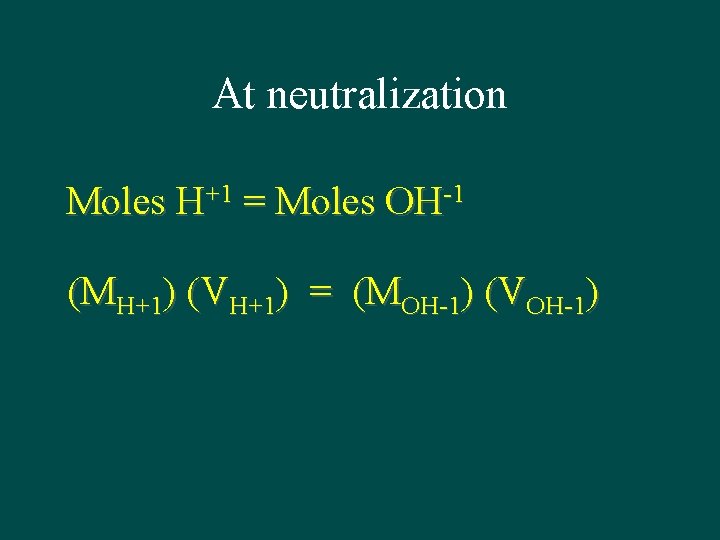
At neutralization Moles H+1 = Moles OH-1 (MH+1) (VH+1) = (MOH-1) (VOH-1)
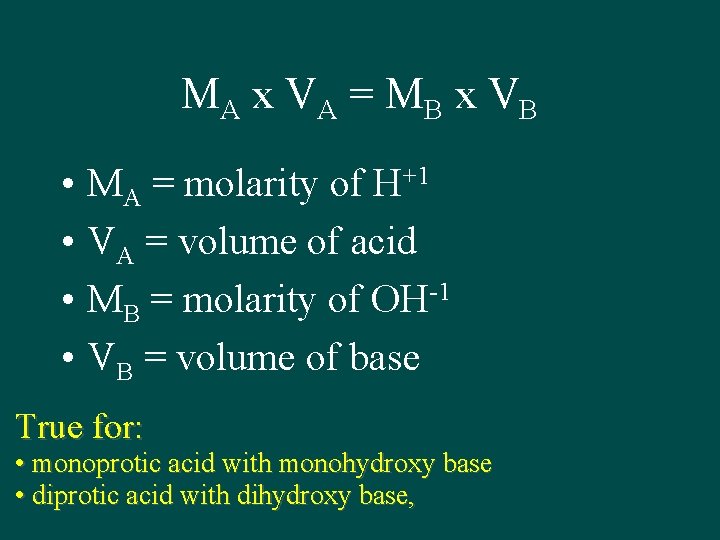
MA x VA = M B x VB • MA = molarity of H+1 • VA = volume of acid • MB = molarity of OH-1 • VB = volume of base True for: • monoprotic acid with monohydroxy base • diprotic acid with dihydroxy base,
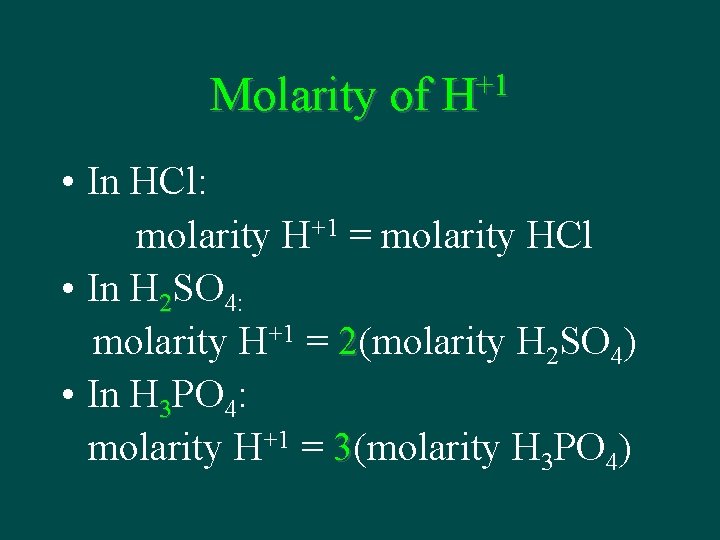
Molarity of +1 H • In HCl: molarity H+1 = molarity HCl • In H 2 SO 4: molarity H+1 = 2(molarity H 2 SO 4) • In H 3 PO 4: molarity H+1 = 3(molarity H 3 PO 4)
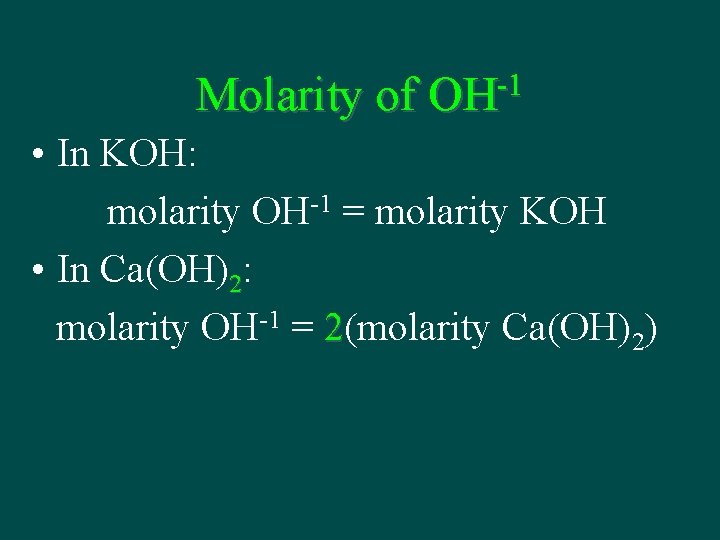
Molarity of -1 OH • In KOH: molarity OH-1 = molarity KOH • In Ca(OH)2: molarity OH-1 = 2(molarity Ca(OH)2)
- Slides: 16