Neutralization reaction Review Whats an acid Example Whats

















- Slides: 26

Neutralization reaction

�Review • What’s an acid? Example? • What’s a base? Example?

�What is neutralization reactio �Neutralization reaction occurs when acid reacts with a base to form a salt General formula: Acid + Base Salt + Water E. g. HCl + Na. OH Na. Cl + HOH Which type of reaction is it? - Double displacement

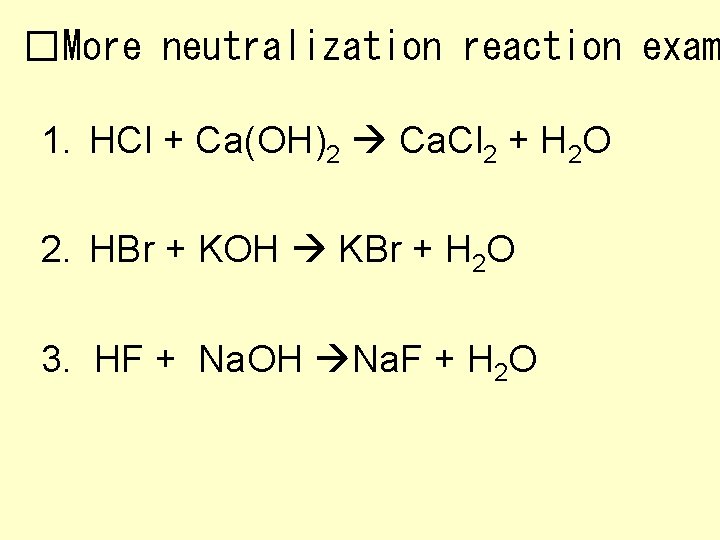
�More neutralization reaction exam 1. HCl + Ca(OH)2 2. HBr + KOH 3. HF + Na. OH

�More neutralization reaction exam 1. HCl + Ca(OH)2 Ca. Cl 2 + H 2 O 2. HBr + KOH KBr + H 2 O 3. HF + Na. OH Na. F + H 2 O

�Applications of Neutralization reactions Our stomach lining ��Antacids are base Mg(OH)2, Al(OH)3) that neutralize excessive stomach acid

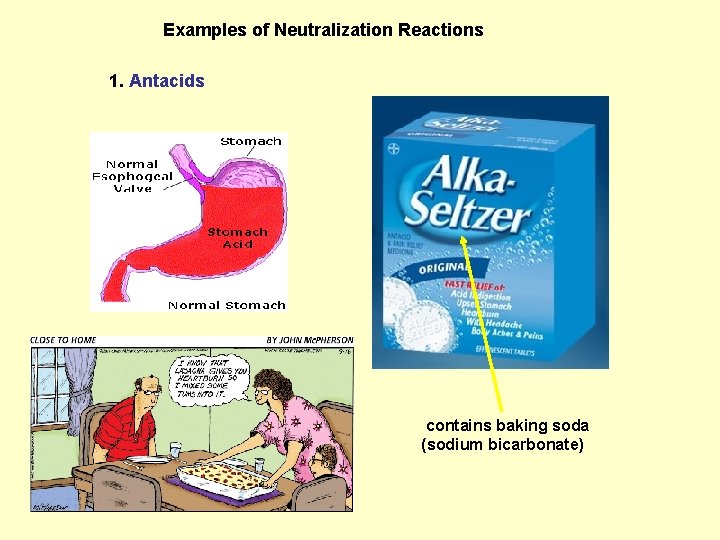
Examples of Neutralization Reactions 1. Antacids contains baking soda (sodium bicarbonate)

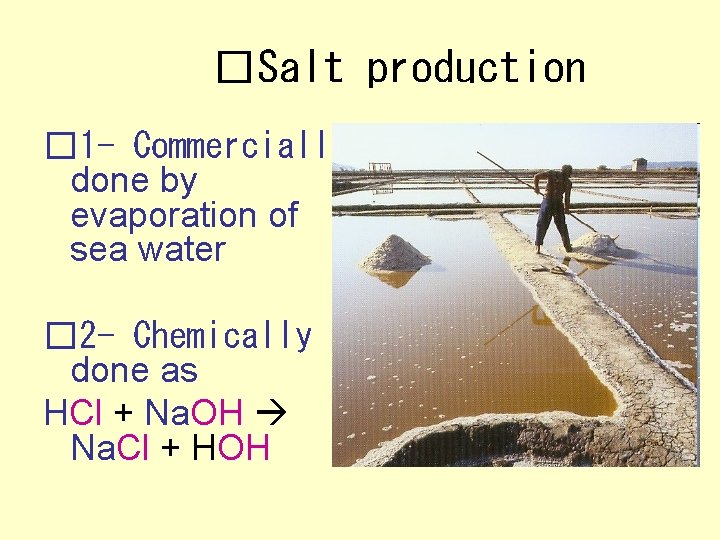
�Salt production � 1 - Commercially done by evaporation of sea water � 2 - Chemically done as HCl + Na. OH Na. Cl + HOH

�Detergent and Soap �What the texture of the base? �Slippery �Because base reacts with the acid of your skin in a neutralization reaction to form soap

�Bee sting When bee stings, formic acid is release Bases should be used to neutralize and reduce pain

What would you do in case of? � �Neutralize H 2 SO 4 with strong bases such as Na. OH or Ca(OH)2

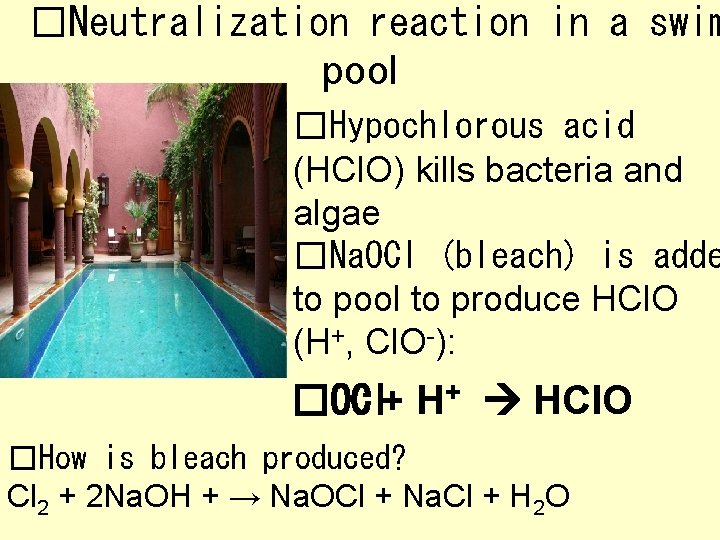
�Neutralization reaction in a swim pool �Hypochlorous acid (HCl. O) kills bacteria and algae �Na. OCl (bleach) is adde to pool to produce HCl. O (H+, Cl. O-): - + H+ HCl. O �OCl �How is bleach produced? Cl 2 + 2 Na. OH + → Na. OCl + Na. Cl + H 2 O

�Neutralization reaction in a swim pool (Cont. ) �If the pool is too acidic, metal pi get corroded. What should be added to neutralize? �� 2 CO Na 3 is added �If the pool is too basic, 3 will form. Ca. CO What should be added to neutralize? �HCl is added

�Hazard with household chemicals �Never mix NH 3 and Na. Cl. O (bleach) NH 3 + Na. Cl. O Na. ONH 3 + Cl 2 �Cl 2 is a very toxic gas, used as weapon during WWI & II �Exposurecan lead to death READ THE LABEL CAREFULLY


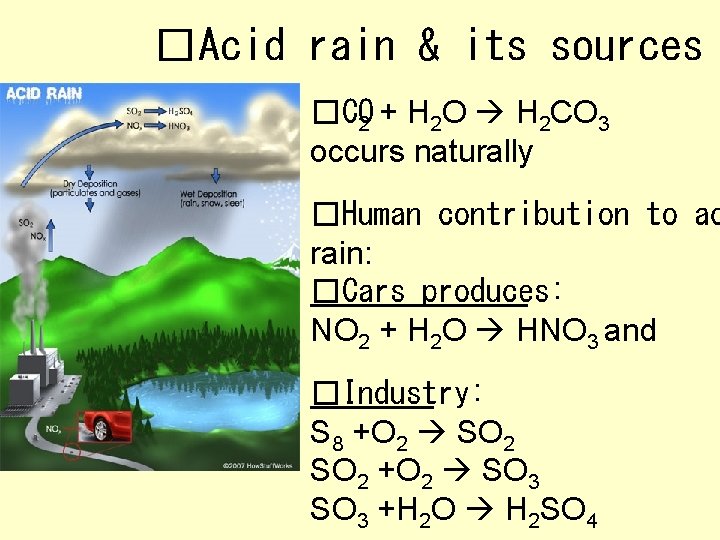
�Acid rain & its sources �CO 2 + H 2 O H 2 CO 3 occurs naturally �Human contribution to ac rain: �Cars produces: NO 2 + H 2 O HNO 3 and �Industry: S 8 +O 2 SO 2 +O 2 SO 3 +H 2 O H 2 SO 4

Acid rain impacts? How to remedy acid rain?

Effects On Forests • Acid rain can have terrible effects on a forest. The acid takes away important minerals from the leaves and the soil. • Minerals are like vitamins for trees and plants. Without them, trees and plants cannot grow properly. They lose their leaves and become very weak. They are no longer strong enough to fight against illnesses and frost. They become very ill and can even die.

Effects On Water Life • Acid rain has a terrible effect on water life. Even if the acid rain does not fall straight into the lake, for example, it may enter from rivers and streams. Some of the life in the lake such as fish and plants may end up dying, because they cannot survive in acidic lakes.

Effects On Fish Acid rain and acid snow are posing a major threat to the fish habitat in Nova Scotia, having already killed one quarter of the province's freshwater fish population. According to scientists, another quarter of the population could be wiped out within twenty years, unless something is done.

Effects On Lakes & Rivers • You can recognize a lake dead from acid rain by its clean and crystal clear water. But they look clean because there is very little living in them anymore. Tiny plants and animals are mostly unable to survive.

Effects On Humans Particulates - very small particles of debris found in some of the air pollution - are one of the main causes of health problems. In towns and cities, these are released mainly by diesel engines from cars and trucks. When we breathe in air pollution, these very fine particulates can easily enter our body, where they can cause breathing problems, and over time even cause cancer. The water we drink from taps can be contaminated by acid rain, which can damage the brain.

Effects On Buildings Acid rain can also ruin buildings because the acid eats into metal and stone. It also damages stained glass and plastics. Some types of building materials are softer than others, and it is the softer ones which are most affected by acid rain. Sandstone and limestone are examples of stone which are fairly soft and are damaged easily. Granite is an example of a harder stone that can resist the effects of acid rain. Buildings are naturally eroded by rain, wind, frost and the sun, but when acidic gases are present, it speeds up the erosion.

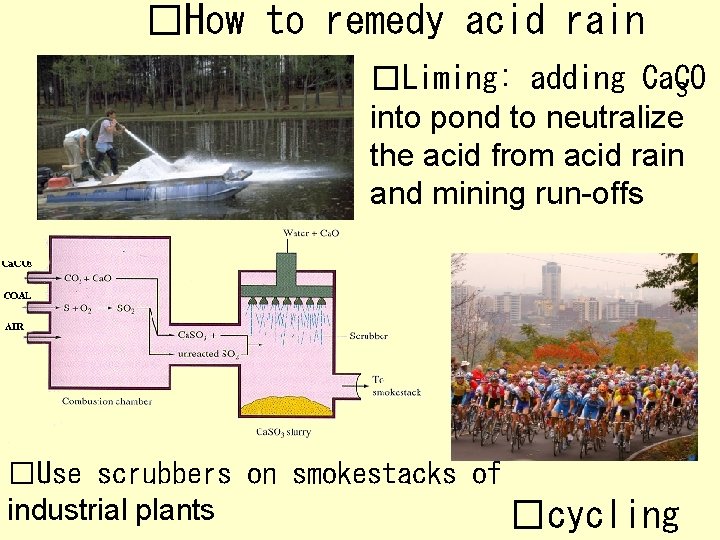
�How to remedy acid rain �Liming: adding Ca. CO 3 into pond to neutralize the acid from acid rain and mining run-offs �Use scrubbers on smokestacks of industrial plants �cycling


Titrations • Standard Solution – The solution of known concentration • Titration – The process of a specific amount of a known solution to an unknown solution to determine the molarity of the unknown solution • End Point – When the indicator shows that neutralization has occurred (color change)