Neutralisation Neutralisation happens when an alkali is mixed








- Slides: 12

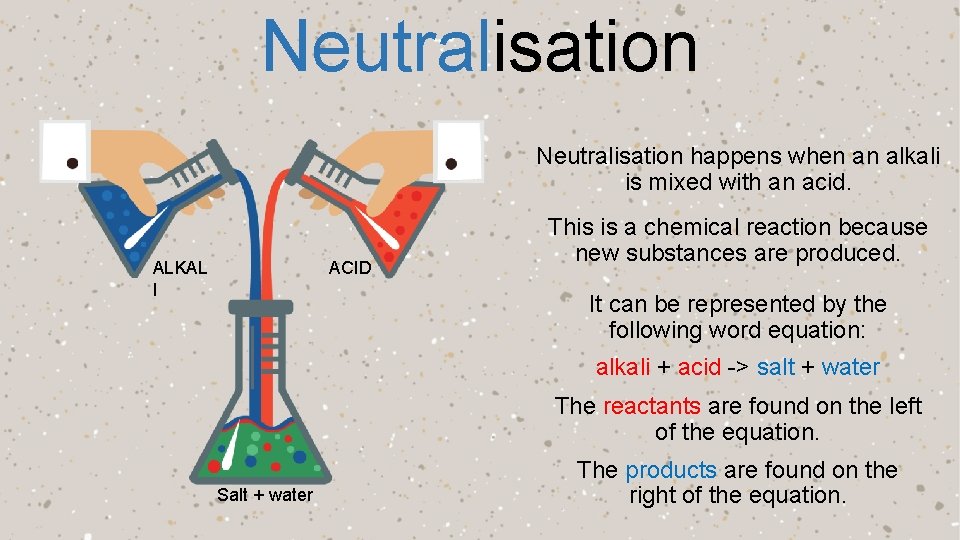
Neutralisation

Neutralisation happens when an alkali is mixed with an acid. ALKAL I ACID This is a chemical reaction because new substances are produced. It can be represented by the following word equation: alkali + acid -> salt + water The reactants are found on the left of the equation. Salt + water The products are found on the right of the equation.

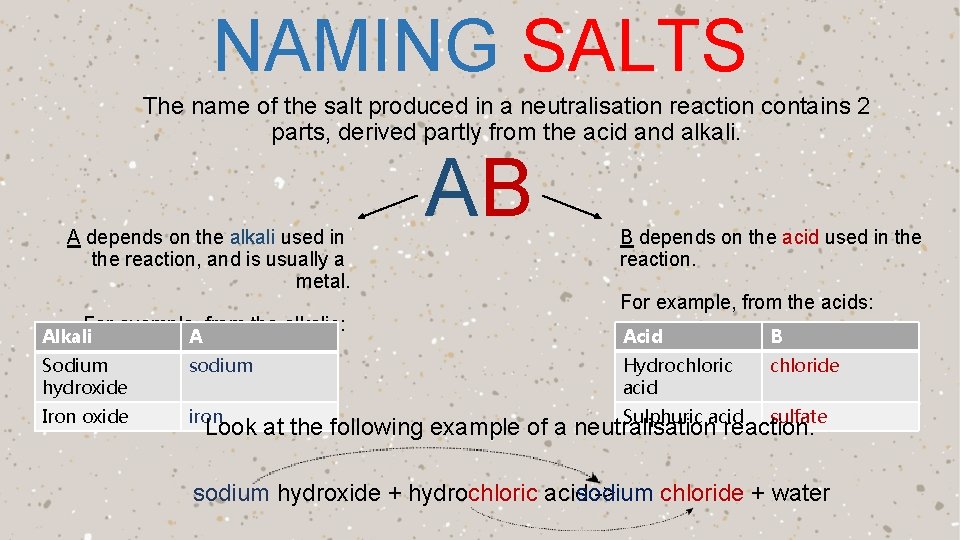
NAMING SALTS The name of the salt produced in a neutralisation reaction contains 2 parts, derived partly from the acid and alkali. A depends on the alkali used in the reaction, and is usually a metal. For example, from the alkalis: AB B depends on the acid used in the reaction. For example, from the acids: Alkali A Acid B Sodium hydroxide sodium Hydrochloric acid chloride Iron oxide iron Sulphuric acid sulfate Look at the following example of a neutralisation reaction. chloride + water sodium hydroxide + hydrochloric acidsodium ->

Can you remember how to Neutralise?

Neutralisation in daily life

#1: Treating a Bee Sting with soap The venom in bee stings contain formic acid and is hence acidic. Because soap is alkaline, adding it to a bee sting will neutralise the acid.

#2: Using Hair conditioner after shampoo Most shampoos are weakly alkaline, and they make our hair unmanageable by causing small scales. Because conditioners are acidic, they neutralise the alkalinity from shampoos and cause the scales to close up.

#3: Treating indigestion with antacid Indigestion occurs when the acid in your stomach returns and irritates the lining of your stomach and food pipe. Antacids (Anti-acids) are alkaline and they help to neutralize the excess acid in the stomach.

#4: Using fluoride toothpaste to prevent cavities When bacteria in the mouth use sugar from food and drink, they produce acids that can dissolve and damage teeth. Fluoride toothpaste is alkaline and using it helps to neutralise the acids, hence preventing cavities.

#5: Using lime fertilisers in soil Over time due to a natural process, soils become acidic. When soils are acidic, they reduce availability of nutrients for plants. By using lime fertilisers, such as powdered lime or limestone, which are all alkaline, it neutralises the soil and increases its p. H.

#6: Cleaning metals with rust removers Metals such as iron and steel can rust over time, forming iron oxide, which is alkaline. Rust removers usually contain phosphoric acid, which reacts with the alkaline rust, forming a black coating which can be easily removed.

What other examples can you think of?