Neuroregulation of Appetite Paleo Nutrition Supports Homeostasis of
- Slides: 1

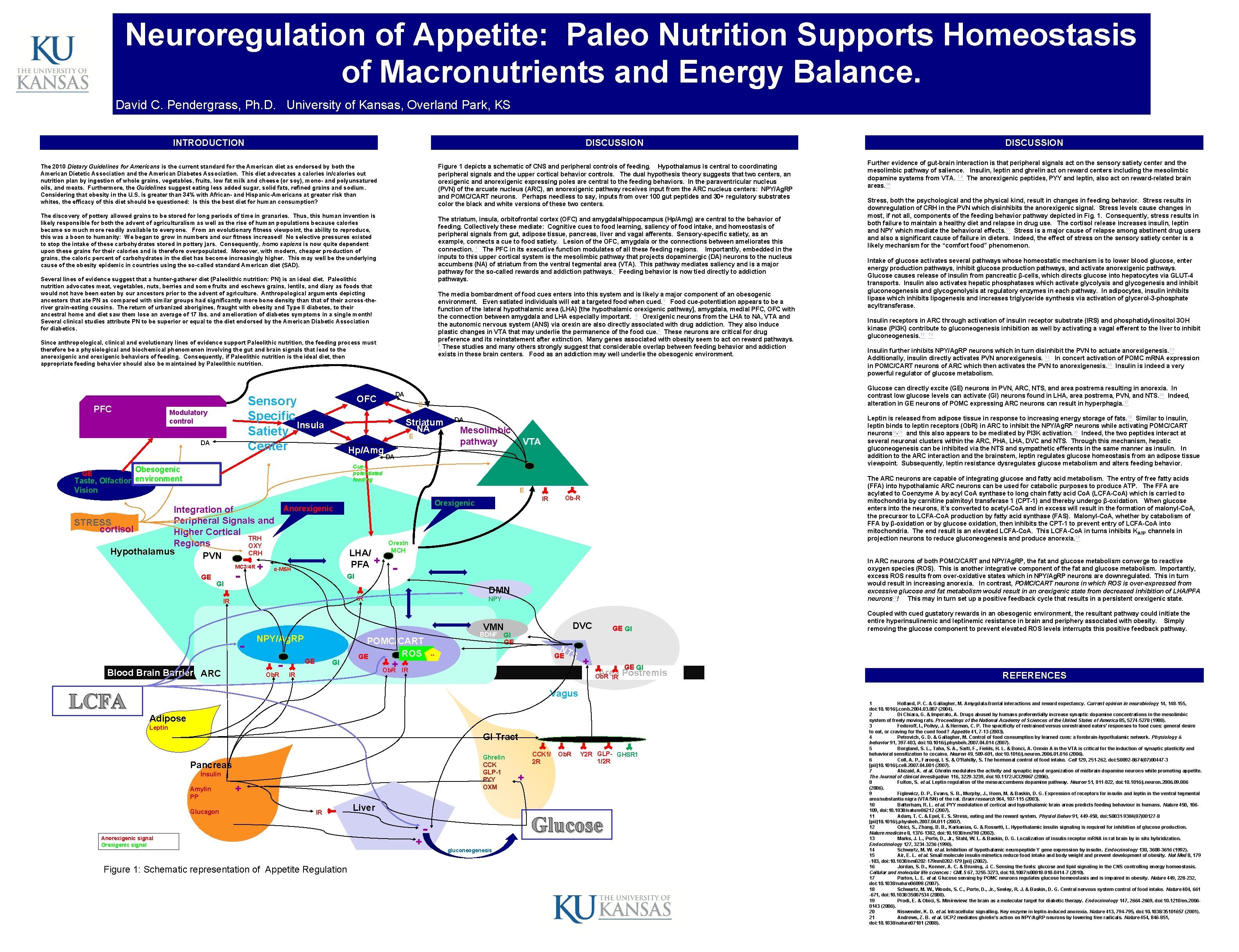
Neuroregulation of Appetite: Paleo Nutrition Supports Homeostasis of Macronutrients and Energy Balance. David C. Pendergrass, Ph. D. University of Kansas, Overland Park, KS INTRODUCTION DISCUSSION Figure 1 depicts a schematic of CNS and peripheral controls of feeding. Hypothalamus is central to coordinating peripheral signals and the upper cortical behavior controls. The dual hypothesis theory suggests that two centers, an orexigenic and anorexigenic expressing poles are central to the feeding behaviors. In the paraventricular nucleus (PVN) of the arcuate nucleus (ARC), an anorexigenic pathway receives input from the ARC nucleus centers: NPY/Ag. RP and POMC/CART neurons. Perhaps needless to say, inputs from over 100 gut peptides and 30+ regulatory substrates color the black and white versions of these two centers. The 2010 Dietary Guidelines for Americans is the current standard for the American diet as endorsed by both the American Dietetic Association and the American Diabetes Association. This diet advocates a calories in/calories out nutrition plan by ingestion of whole grains, vegetables, fruits, low fat milk and cheese (or soy), mono- and polyunsatured oils, and meats. Furthermore, the Guidelines suggest eating less added sugar, solid fats, refined grains and sodium. Considering that obesity in the U. S. is greater than 34% with African- and Hispanic-Americans at greater risk than whites, the efficacy of this diet should be questioned: Is this the best diet for human consumption? The discovery of pottery allowed grains to be stored for long periods of time in granaries. Thus, this human invention is likely responsible for both the advent of agriculturalism as well as the rise of human populations because calories became so much more readily available to everyone. From an evolutionary fitness viewpoint, the ability to reproduce, this was a boon to humanity: We began to grow in numbers and our fitness increased! No selective pressures existed to stop the intake of these carbohydrates stored in pottery jars. Consequently, homo sapiens is now quite dependent upon these grains for their calories and is therefore overpopulated. Moreover, with modern, cheaper production of grains, the caloric percent of carbohydrates in the diet has become increasingly higher. This may well be the underlying cause of the obesity epidemic in countries using the so-called standard American diet (SAD). The striatum, insula, orbitofrontal cortex (OFC) and amygdala/hippocampus (Hp/Amg) are central to the behavior of feeding. Collectively these mediate: Cognitive cues to food learning, saliency of food intake, and homeostasis of peripheral signals from gut, adipose tissue, pancreas, liver and vagal afferents. Sensory-specific satiety, as an example, connects a cue to food satiety. Lesion of the OFC, amygdala or the connections between ameliorates this connection. 1 The PFC in its executive function modulates of all these feeding regions. Importantly, embedded in the inputs to this upper cortical system is the mesolimbic pathway that projects dopaminergic (DA) neurons to the nucleus accumbens (NA) of striatum from the ventral tegmental area (VTA). This pathway mediates saliency and is a major pathway for the so-called rewards and addiction pathways. 2 Feeding behavior is now tied directly to addiction pathways. Several lines of evidence suggest that a hunter-gatherer diet (Paleolithic nutrition: PN) is an ideal diet. Paleolithic nutrition advocates meat, vegetables, nuts, berries and some fruits and eschews grains, lentils, and diary as foods that would not have been eaten by our ancestors prior to the advent of agriculture. Anthropological arguments depicting ancestors that ate PN as compared with similar groups had significantly more bone density than that of their across-theriver grain-eating cousins. The return of urbanized aborigines, fraught with obesity and Type II diabetes, to their ancestral home and diet saw them lose an average of 17 lbs. and amelioration of diabetes symptoms in a single month! Several clinical studies attribute PN to be superior or equal to the diet endorsed by the American Diabetic Association for diabetics. The media bombardment of food cues enters into this system and is likely a major component of an obesogenic environment. Even satiated individuals will eat a targeted food when cued. 3 Food cue-potentiation appears to be a function of the lateral hypothalamic area (LHA) [the hypothalamic orexigenic pathway], amygdala, medial PFC, OFC with the connection between amygdala and LHA especially important. 4 Orexigenic neurons from the LHA to NA, VTA and the autonomic nervous system (ANS) via orexin are also directly associated with drug addiction. They also induce plastic changes in VTA that may underlie the permanence of the food cue. 5 These neurons are critical for drug preference and its reinstatement after extinction. Many genes associated with obesity seem to act on reward pathways. 6 These studies and many others strongly suggest that considerable overlap between feeding behavior and addiction exists in these brain centers. Food as an addiction may well underlie the obesogenic environment. Since anthropological, clinical and evolutionary lines of evidence support Paleolithic nutrition, the feeding process must therefore be a physiological and biochemical phenomenon involving the gut and brain signals that lead to the anorexigenic and orexigenic behaviors of feeding. Consequently, if Paleolithic nutrition is the ideal diet, then appropriate feeding behavior should also be maintained by Paleolithic nutrition. PFC Sensory Specific Insula Satiety Center Modulatory control DA E Striatum NA E Hp/Amg DA Mesolimbic pathway DA Cuepotentiated feeding Obesogenic GE Taste, Olfaction, environment Vision E MC 3/4 R GE GI - + LHA/ PFA + α-MSH GI - Fig. 1 Figure 1 Orexin MCH DMN IR IR NPY/Ag. RP Blood Brain Barrier ARC Ob. R GI NPY POMC/CART ROS GE -Median Eminence GE IR Ob-R IR Orexigenic Anorexigenic Integration of Peripheral Signals and STRESS cortisol Higher Cortical TRH Regions OXY Hypothalamus CRH PVN LCFA DA OFC + Ob. R Figure 1 DVC VMN GE GI BDNF GI GE NT GE ** S IR + GE GI Area Postremis Ob. R IR DISCUSSION Further evidence of gut-brain interaction is that peripheral signals act on the sensory satiety center and the mesolimbic pathway of salience. Insulin, leptin and ghrelin act on reward centers including the mesolimbic dopamine systems from VTA. 7 -9 The anorexigenic peptides, PYY and leptin, also act on reward-related brain areas. 10 Stress, both the psychological and the physical kind, result in changes in feeding behavior. Stress results in downregulation of CRH in the PVN which disinhibits the anorexigenic signal. Stress levels cause changes in most, if not all, components of the feeding behavior pathway depicted in Fig. 1. Consequently, stress results in both failure to maintain a healthy diet and relapse in drug use. The cortisol release increases insulin, leptin and NPY which mediate the behavioral effects. 11 Stress is a major cause of relapse among abstinent drug users and also a significant cause of failure in dieters. Indeed, the effect of stress on the sensory satiety center is a likely mechanism for the “comfort food” phenomenon. Intake of glucose activates several pathways whose homeostatic mechanism is to lower blood glucose, enter energy production pathways, inhibit glucose production pathways, and activate anorexigenic pathways. Glucose causes release of insulin from pancreatic β-cells, which directs glucose into hepatocytes via GLUT-4 transports. Insulin also activates hepatic phosphatases which activate glycolysis and glycogenesis and inhibit gluconeogenesis and glycogenolysis at regulatory enzymes in each pathway. In adipocytes, insulin inhibits lipase which inhibits lipogenesis and increases triglyceride synthesis via activation of glycerol-3 -phosphate acyltransferase. Insulin receptors in ARC through activation of insulin receptor substrate (IRS) and phosphatidylinositol 3 OH kinase (PI 3 K) contribute to gluconeogenesis inhibition as well by activating a vagal efferent to the liver to inhibit gluconeogenesis. 12 13 Insulin further inhibits NPY/Ag. RP neurons which in turn disinhibit the PVN to actuate anorexigenesis. 14 Additionally, insulin directly activates PVN anorexigenesis. 13 In concert activation of POMC m. RNA expression in POMC/CART neurons of ARC which then activates the PVN to anorexigenesis. 15 Insulin is indeed a very powerful regulator of glucose metabolism. Glucose can directly excite (GE) neurons in PVN, ARC, NTS, and area postrema resulting in anorexia. In contrast low glucose levels can activate (GI) neurons found in LHA, area postrema, PVN, and NTS. 16 Indeed, alteration in GE neurons of POMC expressing ARC neurons can result in hyperphagia. 17 Leptin is released from adipose tissue in response to increasing energy storage of fats. 18 Similar to insulin, leptin binds to leptin receptors (Ob. R) in ARC to inhibit the NPY/Ag. RP neurons while activating POMC/CART neurons 18, 19 and this also appears to be mediated by PI 3 K activation. 20 Indeed, the two peptides interact at several neuronal clusters within the ARC, PHA, LHA, DVC and NTS. Through this mechanism, hepatic gluconeogenesis can be inhibited via the NTS and sympathetic efferents in the same manner as insulin. In addition to the ARC interaction and the brainstem, leptin regulates glucose homeostasis from an adipose tissue viewpoint. Subsequently, leptin resistance dysregulates glucose metabolism and alters feeding behavior. The ARC neurons are capable of integrating glucose and fatty acid metabolism. The entry of free fatty acids (FFA) into hypothalamic ARC neurons can be used for catabolic purposes to produce ATP. The FFA are acylated to Coenzyme A by acyl Co. A synthase to long chain fatty acid Co. A (LCFA-Co. A) which is carried to mitochondria by carnitine palmitoyl transferase 1 (CPT-1) and thereby undergo β-oxidation. When glucose enters into the neurons, it’s converted to acetyl-Co. A and in excess will result in the formation of malonyl-Co. A, the precursor to LCFA-Co. A production by fatty acid synthase (FAS). Malonyl-Co. A, whether by catabolism of FFA by β-oxidation or by glucose oxidation, then inhibits the CPT-1 to prevent entry of LCFA-Co. A into mitochondria. The end result is an elevated LCFA-Co. A. This LCFA-Co. A in turns inhibits K ATP channels in projection neurons to reduce gluconeogenesis and produce anorexia. 16 In ARC neurons of both POMC/CART and NPY/Ag. RP, the fat and glucose metabolism converge to reactive oxygen species (ROS). This is another integrative component of the fat and glucose metabolism. Importantly, excess ROS results from over-oxidative states which in NPY/Ag. RP neurons are downregulated. This in turn would result in increasing anorexia. In contrast, POMC/CART neurons in which ROS is over-expressed from excessive glucose and fat metabolism would result in an orexigenic state from decreased inhibition of LHA/PFA neurons 21! This may in turn set up a positive feedback cycle that results in a persistent orexigenic state. Coupled with cued gustatory rewards in an obesogenic environment, the resultant pathway could initiate the entire hyperinsulinemic and leptinemic resistance in brain and periphery associated with obesity. Simply removing the glucose component to prevent elevated ROS levels interrupts this positive feedback pathway. REFERENCES Vagus Adipose Leptin GI Tract Ghrelin CCK GLP-1 PYY OXM Pancreas Insulin Amylin PP Glucagon + IR Anorexigenic signal Orexigenic signal Figure 1: Schematic representation of Appetite Regulation Liver + CCK 1/ 2 R Ob. R Y 2 R GLP- GHSR 1 1/2 R + Glucose gluconeogenesis 1 Holland, P. C. & Gallagher, M. Amygdala-frontal interactions and reward expectancy. Current opinion in neurobiology 14, 148 -155, doi: 10. 1016/j. conb. 2004. 03. 007 (2004). 2 Di Chiara, G. & Imperato, A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proceedings of the National Academy of Sciences of the United States of America 85, 5274 -5278 (1988). 3 Fedoroff, I. , Polivy, J. & Herman, C. P. The specificity of restrained versus unrestrained eaters' responses to food cues: general desire to eat, or craving for the cued food? Appetite 41, 7 -13 (2003). 4 Petrovich, G. D. & Gallagher, M. Control of food consumption by learned cues: a forebrain-hypothalamic network. Physiology & behavior 91, 397 -403, doi: 10. 1016/j. physbeh. 2007. 04. 014 (2007). 5 Borgland, S. L. , Taha, S. A. , Sarti, F. , Fields, H. L. & Bonci, A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron 49, 589 -601, doi: 10. 1016/j. neuron. 2006. 016 (2006). 6 Coll, A. P. , Farooqi, I. S. & O'Rahilly, S. The hormonal control of food intake. Cell 129, 251 -262, doi: S 0092 -8674(07)00447 -3 [pii]10. 1016/j. cell. 2007. 04. 001 (2007). 7 Abizaid, A. et al. Ghrelin modulates the activity and synaptic input organization of midbrain dopamine neurons while promoting appetite. The Journal of clinical investigation 116, 3229 -3239, doi: 10. 1172/JCI 29867 (2006). 8 Fulton, S. et al. Leptin regulation of the mesoaccumbens dopamine pathway. Neuron 51, 811 -822, doi: 10. 1016/j. neuron. 2006. 09. 006 (2006). 9 Figlewicz, D. P. , Evans, S. B. , Murphy, J. , Hoen, M. & Baskin, D. G. Expression of receptors for insulin and leptin in the ventral tegmental area/substantia nigra (VTA/SN) of the rat. Brain research 964, 107 -115 (2003). 10 Batterham, R. L. et al. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature 450, 106109, doi: 10. 1038/nature 06212 (2007). 11 Adam, T. C. & Epel, E. S. Stress, eating and the reward system. Physiol Behav 91, 449 -458, doi: S 0031 -9384(07)00127 -8 [pii]10. 1016/j. physbeh. 2007. 04. 011 (2007). 12 Obici, S. , Zhang, B. B. , Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nature medicine 8, 1376 -1382, doi: 10. 1038/nm 798 (2002). 13 Marks, J. L. , Porte, D. , Jr. , Stahl, W. L. & Baskin, D. G. Localization of insulin receptor m. RNA in rat brain by in situ hybridization. Endocrinology 127, 3234 -3236 (1990). 14 Schwartz, M. W. et al. Inhibition of hypothalamic neuropeptide Y gene expression by insulin. Endocrinology 130, 3608 -3616 (1992). 15 Air, E. L. et al. Small molecule insulin mimetics reduce food intake and body weight and prevent development of obesity. Nat Med 8, 179 -183, doi: 10. 1038/nm 0202 -179 [pii] (2002). 16 Jordan, S. D. , Konner, A. C. & Bruning, J. C. Sensing the fuels: glucose and lipid signaling in the CNS controlling energy homeostasis. Cellular and molecular life sciences : CMLS 67, 3255 -3273, doi: 10. 1007/s 00018 -010 -0414 -7 (2010). 17 Parton, L. E. et al. Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449, 228 -232, doi: 10. 1038/nature 06098 (2007). 18 Schwartz, M. W. , Woods, S. C. , Porte, D. , Jr. , Seeley, R. J. & Baskin, D. G. Central nervous system control of food intake. Nature 404, 661 -671, doi: 10. 1038/35007534 (2000). 19 Prodi, E. & Obici, S. Minireview: the brain as a molecular target for diabetic therapy. Endocrinology 147, 2664 -2669, doi: 10. 1210/en. 20060143 (2006). 20 Niswender, K. D. et al. Intracellular signalling. Key enzyme in leptin-induced anorexia. Nature 413, 794 -795, doi: 10. 1038/35101657 (2001). 21 Andrews, Z. B. et al. UCP 2 mediates ghrelin's action on NPY/Ag. RP neurons by lowering free radicals. Nature 454, 846 -851, doi: 10. 1038/nature 07181 (2008).