Neuromodulation Device Market Innovations and Trends Presenters Chrystal


















- Slides: 35

Neuromodulation Device Market – Innovations and Trends

Presenters Chrystal Larsen Lead Market Analyst - Meddevicetracker Marion Webb Managing Editor - Medtech Insight David Filmore Editor in Chief - Medtech Insight 2 Pharma intelligence | informa

Agenda • Introduction • Overview of neuromodulation • Neuromodulation Market Overview • Market size • Market forecast /drivers & limiters • Competitors/market share • Technology trends driving growth • PNS Overview • Trends, key innovations • US Policy Developments • More support for neuromod? • 3 Q&A Pharma intelligence | informa

Overview – Neuromodulation 4 • Neuromodulation (also termed “neurostimulation”) uses implantable devices (battery-powered implantable pulse generators or IPGs and electrical leads) to stimulate targeted nerves. • Goal: modulate abnormal nerve activity, alleviate debilitating pain or neurological symptoms (tremor, seizures, etc). • Pioneered by Medtronic (invented DBS, SCS). • In use since the late 1960 s; commercial use since the 1970 s-80 s; majority of growth past 5 -7 years. • Indication: severe, intractable cases when traditional medication/surgery fails. Pharma intelligence | informa

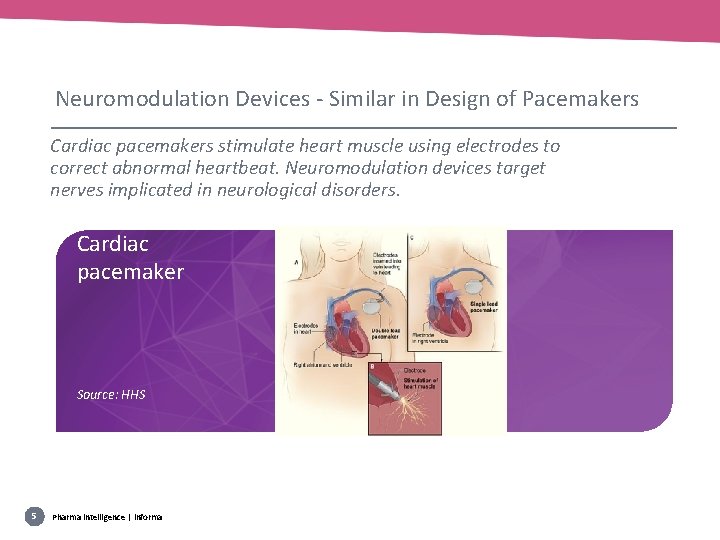
Neuromodulation Devices - Similar in Design of Pacemakers Cardiac pacemakers stimulate heart muscle using electrodes to correct abnormal heartbeat. Neuromodulation devices target nerves implicated in neurological disorders. Cardiac pacemaker Source: HHS 5 Pharma intelligence | informa

Largest Neuromodulation Segments Top 4 segments*: 1) spinal cord stimulation (SCS) 2) deep brain stimulation (DBS) 3) vagus nerve stimulation (VNS) 4) sacral nerve stimulation (SNS) *Note: For the purposes of this webinar, we are focusing on SCS/DBS/VNS. Excluding SNS, currently monopolized by Medtronic (Inter. Stim & NURO systems for OAB) and cochlear implants for hearing loss. 6 Pharma intelligence | informa

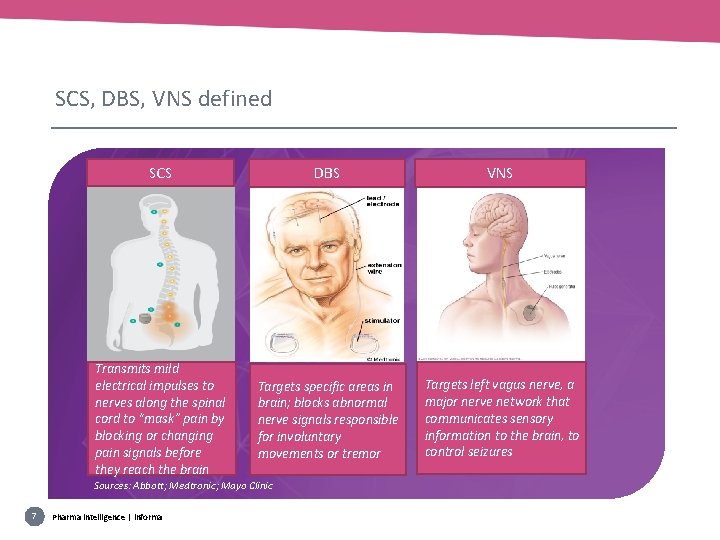
SCS, DBS, VNS defined SCS DBS VNS Transmits mild electrical impulses to nerves along the spinal cord to “mask” pain by blocking or changing pain signals before they reach the brain Targets specific areas in brain; blocks abnormal nerve signals responsible for involuntary movements or tremor Targets left vagus nerve, a major nerve network that communicates sensory information to the brain, to control seizures Sources: Abbott; Medtronic; Mayo Clinic 7 Pharma intelligence | informa

Prevalence SCS: 1. 5 billion people suffer from chronic pain worldwide • 100 million chronic pain sufferers in the US; 30 million from chronic back pain (US) • addresses opioid epidemic in US: 100 people die every day from opioid overdose DBS: Parkinson’s disease: 10 million worldwide; 1. 1 million (US) • Essential tremor: 7 -10 million (US) • Dystonia: <300, 000 (US) VNS: epilepsy: 50 -65 million worldwide; 3. 4 million (US) • 8 Drug-refractory epilepsy: 1 million (US) Pharma intelligence | informa Source: See Meddevicetracker’s reports cited at end of webinar: Spinal Cord Stimulation Devices Market and Deep Brain Stimulation & Vagus Nerve Stimulation Devices Market

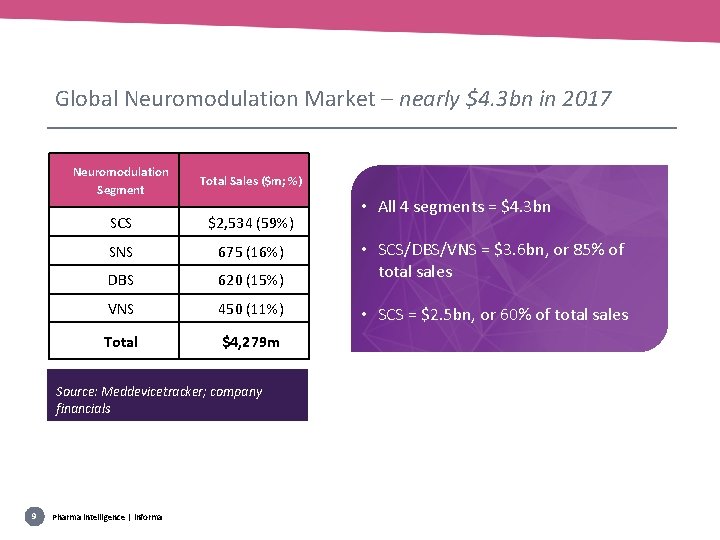
Global Neuromodulation Market – nearly $4. 3 bn in 2017 Neuromodulation Segment Total Sales ($m; %) SCS $2, 534 (59%) SNS 675 (16%) DBS 620 (15%) • SCS/DBS/VNS = $3. 6 bn, or 85% of total sales VNS 450 (11%) • SCS = $2. 5 bn, or 60% of total sales Total $4, 279 m Source: Meddevicetracker; company financials 9 Pharma intelligence | informa • All 4 segments = $4. 3 bn

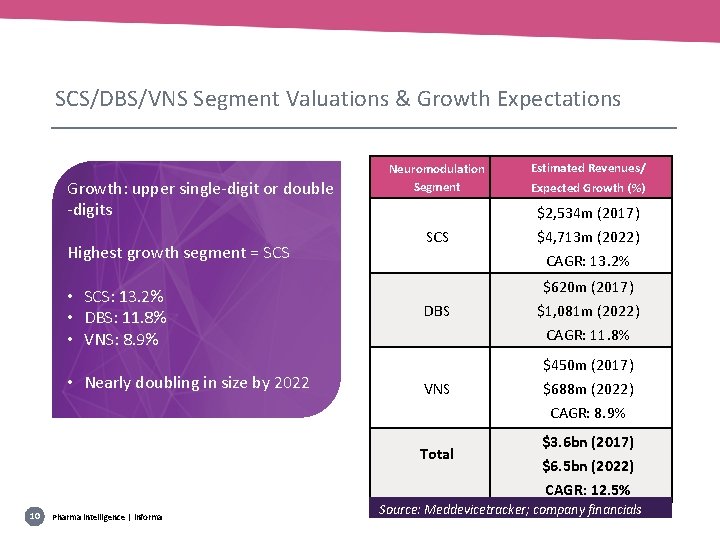
SCS/DBS/VNS Segment Valuations & Growth Expectations Growth: upper single-digit or double -digits Highest growth segment = SCS • SCS: 13. 2% • DBS: 11. 8% • VNS: 8. 9% • Nearly doubling in size by 2022 Neuromodulation Segment Estimated Revenues/ Expected Growth (%) $2, 534 m (2017) SCS $4, 713 m (2022) CAGR: 13. 2% $620 m (2017) DBS $1, 081 m (2022) CAGR: 11. 8% $450 m (2017) VNS $688 m (2022) CAGR: 8. 9% Total $3. 6 bn (2017) $6. 5 bn (2022) CAGR: 12. 5% 10 Pharma intelligence | informa Source: Meddevicetracker; company financials

Market Drivers & Limiters Market Drivers • Aging population affected by chronic pain & incurable neurological disorders • Growing opioid epidemic & need for non -opioid alternatives • Large SCS segment driving growth • DBS/VNS also growing • Good clinical history • Innovation driving growth • Low penetration • Increasing awareness/acceptance • Good/expanding surgeon networks • Strong marketing, distribution, training • Expanding indications 11 Pharma intelligence | informa Market Limiters • Patient reluctance for implants/surgery • Risks • Surgical complexity/surgeon learning curve • Second battery-replacement surgery • High cost • Regulatory restrictions OUS • Heavy competition/pricing pressures • Lack of long-term clinical studies comparing systems/new indications

Competitive Landscape – Market Leaders SCS DBS VNS 12 Pharma intelligence | informa

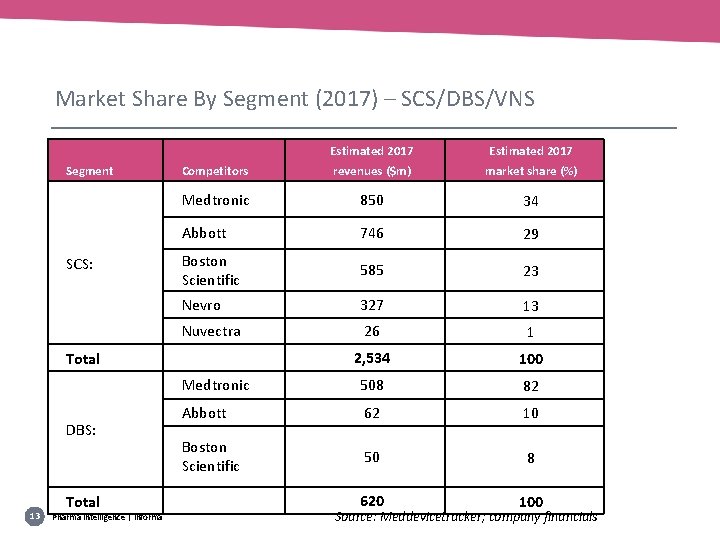
Market Share By Segment (2017) – SCS/DBS/VNS Segment SCS: Estimated 2017 Competitors revenues ($m) market share (%) Medtronic 850 34 Abbott 746 29 Boston Scientific 585 23 Nevro 327 13 Nuvectra 26 1 2, 534 100 Medtronic 508 82 Abbott 62 10 50 8 620 100 Total DBS: 13 Source: Meddevicetracker; company financials Total Pharma intelligence | informa Boston Scientific Source: Meddevicetracker; company financials

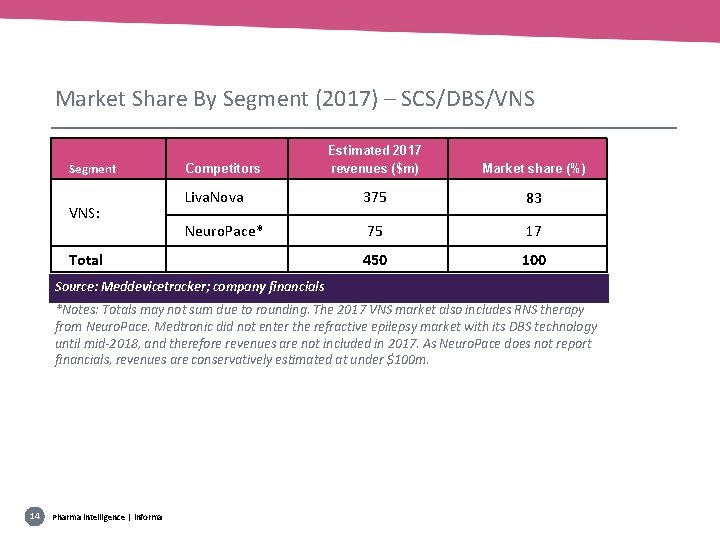
Market Share By Segment (2017) – SCS/DBS/VNS Segment VNS: Estimated 2017 revenues ($m) Market share (%) Liva. Nova 375 83 Neuro. Pace* 75 17 450 100 Competitors Total Source: Meddevicetracker; company financials *Notes: Totals may not sum due to rounding. The 2017 VNS market also includes RNS therapy from Neuro. Pace. Medtronic did not enter the refractive epilepsy market with its DBS technology until mid-2018, and therefore revenues are not included in 2017. As Neuro. Pace does not report financials, revenues are conservatively estimated at under $100 m. 14 Pharma intelligence | informa

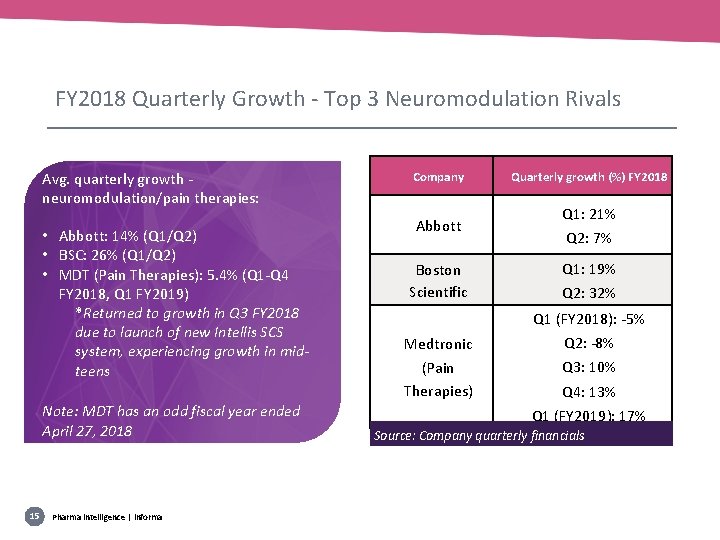
FY 2018 Quarterly Growth - Top 3 Neuromodulation Rivals Avg. quarterly growth neuromodulation/pain therapies: • Abbott: 14% (Q 1/Q 2) • BSC: 26% (Q 1/Q 2) • MDT (Pain Therapies): 5. 4% (Q 1 -Q 4 FY 2018, Q 1 FY 2019) *Returned to growth in Q 3 FY 2018 due to launch of new Intellis SCS system, experiencing growth in midteens Note: MDT has an odd fiscal year ended April 27, 2018 15 Pharma intelligence | informa Company Abbott Boston Scientific Quarterly growth (%) FY 2018 Q 1: 21% Q 2: 7% Q 1: 19% Q 2: 32% Q 1 (FY 2018): -5% Medtronic Q 2: -8% (Pain Therapies) Q 3: 10% Q 4: 13% Q 1 (FY 2019): 17% Source: Company quarterly financials

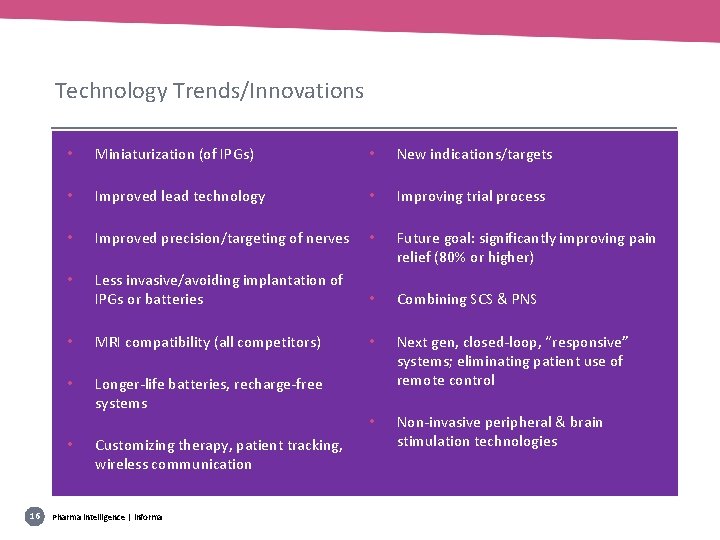
Technology Trends/Innovations • Miniaturization (of IPGs) • New indications/targets • Improved lead technology • Improving trial process • Improved precision/targeting of nerves • Future goal: significantly improving pain relief (80% or higher) • Less invasive/avoiding implantation of IPGs or batteries • Combining SCS & PNS • MRI compatibility (all competitors) • • Longer-life batteries, recharge-free systems Next gen, closed-loop, “responsive” systems; eliminating patient use of remote control • Non-invasive peripheral & brain stimulation technologies • 16 Customizing therapy, patient tracking, wireless communication Pharma intelligence | informa

Abbott’s Burst. DR stimulation (SCS) & new Proclaim DRG system Proclaim Elite Recharge-free SCS System • Proprietary Burst. DR stimulation waveform technology • Both paresthesia & paresthesia-free (no tingling sensation) • Clinically proven to provide superior pain relief vs. traditional SCS (SUNBURST study; over 50% reduction in pain and high patient satisfaction for Burst. DR) Proclaim DRG Neurostimulation System • FDA approved October 2017 • First neuromodulation device approved for treating complex regional pain syndrome types I and II 17 Pharma intelligence | informa

Boston Scientific’s New Spectra Wavewriter for SCS • FDA approved January 2018 • Offers both paresthesia & subperception (no paresthesia) • First SCS system with Waveform Automation • First SCS system with Contour Field. Shaping • Customized to each patient’s spinal anatomy for superior pain relief 18 Pharma intelligence | informa

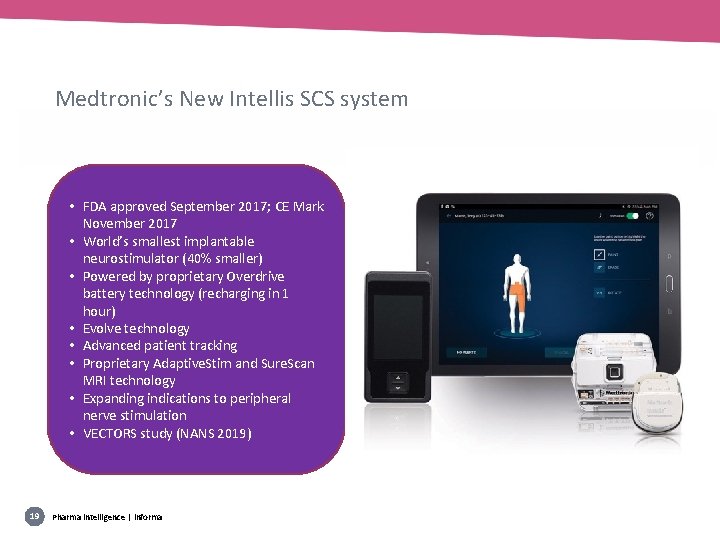
Medtronic’s New Intellis SCS system • FDA approved September 2017; CE Mark November 2017 • World’s smallest implantable neurostimulator (40% smaller) • Powered by proprietary Overdrive battery technology (recharging in 1 hour) • Evolve technology • Advanced patient tracking • Proprietary Adaptive. Stim and Sure. Scan MRI technology • Expanding indications to peripheral nerve stimulation • VECTORS study (NANS 2019) 19 Pharma intelligence | informa

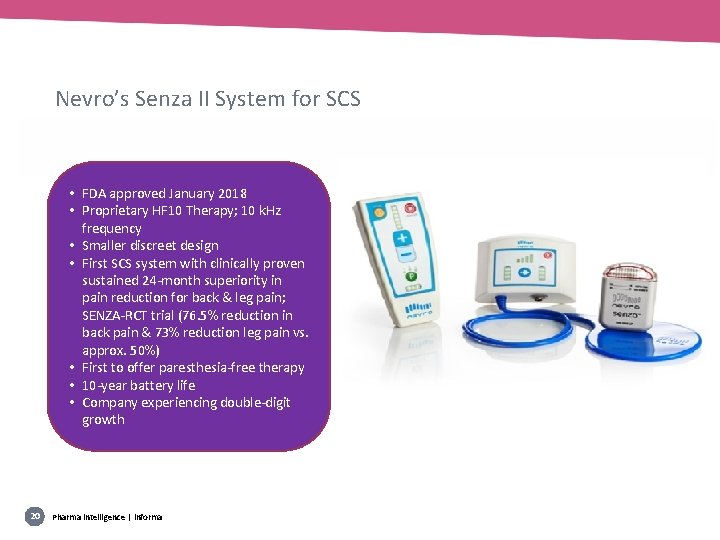
Nevro’s Senza II System for SCS • FDA approved January 2018 • Proprietary HF 10 Therapy; 10 k. Hz frequency • Smaller discreet design • First SCS system with clinically proven sustained 24 -month superiority in pain reduction for back & leg pain; SENZA-RCT trial (76. 5% reduction in back pain & 73% reduction leg pain vs. approx. 50%) • First to offer paresthesia-free therapy • 10 -year battery life • Company experiencing double-digit growth 20 Pharma intelligence | informa

New Directional Lead Technology - DBS (Abbott & Boston Scientific) Abbott’s Infinity DBS System • First FDA-approved proprietary Directional Lead technology • Allows more precise targeting/fewer side effects • Lead: designed w/segmented electrodes, individually controlled • Smallest non-rechargeable battery • First DBS system with wireless connectivity • Clinicals/PD= doubled “on-time”(4. 27 hrs vs 1. 77 hrs) & 90% rated good-excellent BSC’s Vercise DBS System • FDA approved Dec. 2017 • Longest rechargeable battery (15 yrs) • Unique MICC lead technology sculpts current field; Cartesia Directional Lead • INTREPID study = 50% improvement motor functions • New Vercise Gevia (CE Mark June 2017); 25 yr rechargeable battery; Stimview software; first fully MRI compatible 21 Pharma intelligence | informa

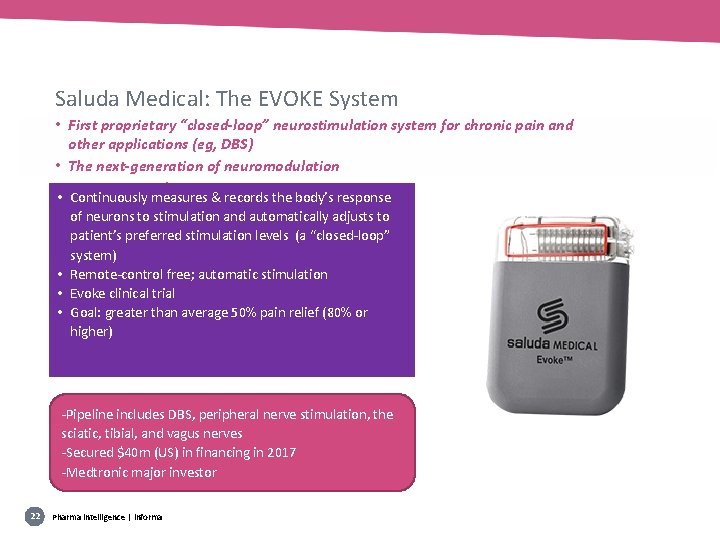
Saluda Medical: The EVOKE System • First proprietary “closed-loop” neurostimulation system for chronic pain and other applications (eg, DBS) • The next-generation of neuromodulation • Investigational • Continuously measures & records the body’s response of neurons to stimulation and automatically adjusts to patient’s preferred stimulation levels (a “closed-loop” system) • Remote-control free; automatic stimulation • Evoke clinical trial • Goal: greater than average 50% pain relief (80% or higher) -Pipeline includes DBS, peripheral nerve stimulation, the sciatic, tibial, and vagus nerves -Secured $40 m (US) in financing in 2017 -Medtronic major investor 22 Pharma intelligence | informa

Brainsway: Deep TMS • Noninvasive Deep Transcranial Magnetic Stimulation (Deep TMS) stimulation device for major depression and other applications • Patents exclusively licensed from the National Institutes of Health (NIH) 23 • FDA approved in May 2018 for major depressive disorder (MDD); FDA approved de novo for OCD (August 2018) • CE Mark approval several indications • Outpatient/no anesthesia/20 -minute sessions • Stimulates deeper & broader neuronal targets in the brain than conventional TMS • Electric field stimulates larger volume of gray matter Pharma intelligence | informa • US pivotal trial response rate of 38. 4% (vs. 21. 4% with sham) after 20 sessions • Over 60 clinical studies; 15, 000 patients treated; good reimbursement

Peripheral Nerve Stimulation (PNS) Overview • PNS development is lagging amid success of SCS • Different from SCS: implant is placed directly over the nerve at targeted area • ACPA: Peripheral neuropathy typically causes pain and numbness in hands and feet, often described as tingling, burning or pricking sensation (paresthesia) or muscle weakness • Causes: Infection, traumatic injury, metabolic disorders, exposure to toxins • More than 100 different types of PN: diabetes common cause 24 Pharma intelligence | informa

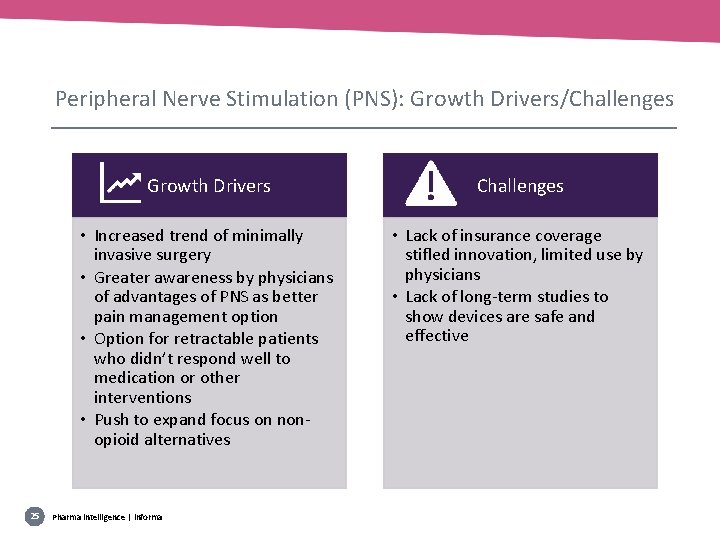
Peripheral Nerve Stimulation (PNS): Growth Drivers/Challenges 25 Growth Drivers Challenges • Increased trend of minimally invasive surgery • Greater awareness by physicians of advantages of PNS as better pain management option • Option for retractable patients who didn’t respond well to medication or other interventions • Push to expand focus on nonopioid alternatives • Lack of insurance coverage stifled innovation, limited use by physicians • Lack of long-term studies to show devices are safe and effective Pharma intelligence | informa

SPR Therapeutics: SPRINT PNS System • First, only US FDA-cleared percutaneous PNS system for treating chronic and acute pain, including postoperative and post-traumatic pain • Next-gen SPRINT endura (single-lead) and extensa (dual-lead) systems FDA approved in August 2018 for 60 -day implantation in the back/extremities • Fills a gap in between opioids and more invasive conventional neuromodulation Features/Benefits: • Threadlike, flexible coiled Micro. Lead is placed percutaneously by physician during out-patient procedure • Lead is connected to small patch-like SPRINT Wearable Stimulator • Delivers neurostimulation for 60 days, can be removed non-surgically Clinical Trials: • Preliminary results multicenter study treating chronic post-amputation pain showed ≥ 50% pain reduction/pain interference in 2/3 rds of subjects after 8 weeks; enduring, significant pain relief ≥ 50% was reported by 4 of 5 patients who completed 12 -month study (data, Napa Pain Conference, August 2018) Funding: • October 2018: $10 m grant US DOD; $30 m total in research government grants and contracts to advance SP • September 2017: $25 m in Series C round led by a “prominent family office” and Frontcourt Ventures 26 Pharma intelligence | informa

Bioness: Stim. Router • US FDA cleared 2015; Canada approved January 2018; CE mark 2014 • Indication: Pain management of severe, intractable chronic pain of peripheral nerve origin as adjunct to other less invasive therapies, such as pain medications • Targets 22 different peripheral nerves around body Features/Benefits: • All electronics are outside the body in wearable transdermal electrical stimulation device, reduces implantation time and anesthesia from 90 to 15 -30 minutes • Battery is implanted underneath skin; SPR battery is external • Lead is implanted through small incision and placed on nerve; backend is external pulse transmitter (EPT), sends electrical signal through skin to lead; programmer patch wirelessly connects to EPT, allows patients to turn stimulation on/off Clinical Trials: • 7 patients implanted with Stim. Router to manage post-stroke shoulder pain, average 70% reduction in chronic pain using visual analog scale (Poster, NANS 2017) Reimbursement: • May 2017, Aetna • February 2018, Highmark Blue Cross Blue Shield 27 Pharma intelligence | informa

electro. Core: gamma. Core • Completely noninvasive, US FDA cleared 2018 for treating acute migraine; April 2017, FDA de novo for treating episodic cluster headaches, CE-marked in Europe 2017 • Treats multiple attacks a day, and up to 4 attacks or 8 tx for a total of 24 stimulations/day Features/Benefits: • Delivers non-invasive vagus nerve stimulation (VNS) to patient’s neck, block signals that cause headache • Vagus nerve, longest cranial nerve in body, brings information from visceral organs to the brain Registry/Clinical Trials: • Electro. Core developed US commercial registry with expert headache centers in US to distribute up to 1, 200 gamma. Core units for free for first 2 months • FDA clearance based on results of 243 -patient PRESTO trial, showed gamma. Core was superior to sham, well tolerated, higher proportion of patients achieved pain relief within 2 hrs vs. control group Funding: • November 2017 completed $70 m Series B funding round led by Core Ventures II, Merck’s Global Health Innovation Fund 28 Pharma intelligence | informa

Neuro. Metrix: Quell TENS unit • Wearable TENS unit used for treating common types of chronic, intractable pain • First FDA-cleared device for treating chronic pain during sleep • Sold OTC without prescription, can be worn 24 hrs a day Features/Benefits: • Two components: an electronic device carried in a neoprene band worn on upper calf and electrode that interfaces the device with the skin • Stimulates nerves to trigger release of body’s own natural pain blockers for widespread pain relief • Designed to relief chronic pain: arthritis, diabetic nerve pain, fibromyalgia, lower back and leg pain Sales Agreement with Glaxo. Smith. Kline : • January 2018, Neuro. Metrix (Waltham, Mass. ) signed agreement with Glaxo. Smith. Kline to expand intl. sales of Quell – Glaxo. Smith. Kline gains exclusive ownership of device for markets outside US; up to $21 m upon in milestones; Neuro. Metrix retains US marketing rights 29 Pharma intelligence | informa

Emerging Technology Mainstay Medical: Re. Activ 8 • Small implantable device to treat chronic low back pain • Differs from SCS systems: electronically stimulates nerves responsible for activating or contracting key stabilizing muscles of the lumbar spine to restore muscle control and stabilize spine over time • Patient uses wireless, handheld remote control 30 min. /2 x a day Features/Benefits: • Implantation of two leads bilaterally near the dorsal ramus nerve at the L 3 vertebra. Leads are connected to a small battery-powered IPG, which generates electrical pulses to dorsal ramus nerves to activate muscles. • External programmer communicates wirelessly with IPG • Customized stimulation by patient Clinical Trials: • Two international, multi-center trials: • Re. Acti 8 -A supported CE mark in May 2016, showed significant and sustained improvement in chronic low back pain • Re. Activ 8 -B, US IDEA trial, 204 patients, data expected year-end for hopeful US approval and in Australia Funding: • In February 2018 raised € 30. 1 m to complete US trial and advance European commercialization 30 Pharma intelligence | informa

US Policy: More Support For Neuromod? • FDA has launched an “Innovation Challenge” Ø Winners announced next month • US Congress recently passed “SUPPORT for Patients and Communities Act” to address the opioid epidemic Ø Further focuses FDA efforts on alternative pain control Ø Medicare provisions, but nothing specific for neuromodulation • More Broadly: CMS has signaled it is working hard to improve the reimbursement process for innovative medical devices 31 Pharma intelligence | informa

Medicare Progress? VNS For Depression 32 • After years of non-coverage, Medicare recently re-opened a National Coverage Analysis for vagus nerve stimulation for Treatment-Resistant Depression. • Based on a request from Liva. Nova, a proposed decision is anticipated next month Pharma intelligence | informa

For detailed market intelligence, see our Meddevicetracker reports: Spinal Cord Stimulation Devices Market: https: //www. meddevicetracker. com/Report. Detail. cfm? Report. ID=372 Deep Brain Stimulation & Vagus Nerve Stimulation Devices Market: https: //www. meddevicetracker. com/Report. Detail. cfm? Report. ID=383 33 Pharma intelligence | informa

Q&A 34 Pharma intelligence | informa

Thank you Chrystal. Larsen@informa. com Marion. Webb@informa. com David. Filmore@informa. com For further questions or to find out more: Email: pharma@informa. com Visit: pharmaintelligence. informa. com