Neuroanatomical Techniques These tell us about the anatomical
Neuroanatomical Techniques • These tell us about the anatomical structure of the brain. • a) Histological Procedures: Gross examination of the brain does not allow us to study details of cell structure and connectivity, to do so we need to selectively stain thin slices of the brain. • Preservation: After death the soft brain tissue is destroyed by autolytic enzymes, so the brain must be preserved with a fixative, such as formalin. • The brain is then embedded within a paraffin block that can be sliced thinly using a microtome and mounted on slides. • Histological stains have been developed so that cell bodies, nerve fibres and membranes can be selectively viewed
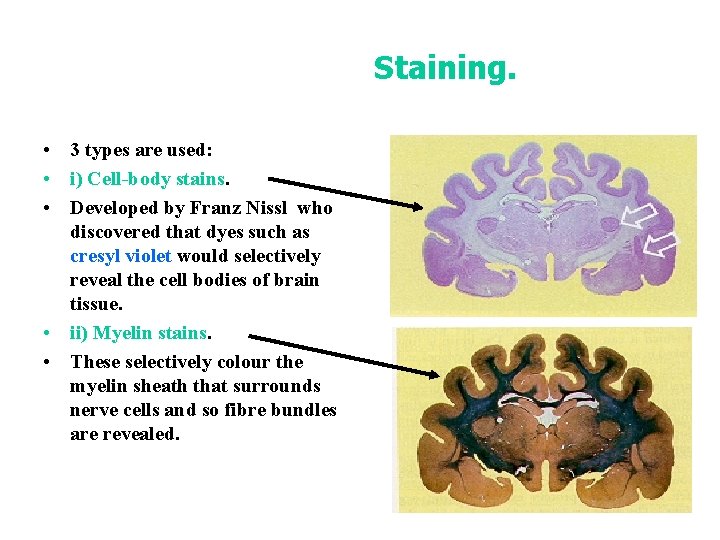
Staining. • 3 types are used: • i) Cell-body stains. • Developed by Franz Nissl who discovered that dyes such as cresyl violet would selectively reveal the cell bodies of brain tissue. • ii) Myelin stains. • These selectively colour the myelin sheath that surrounds nerve cells and so fibre bundles are revealed.
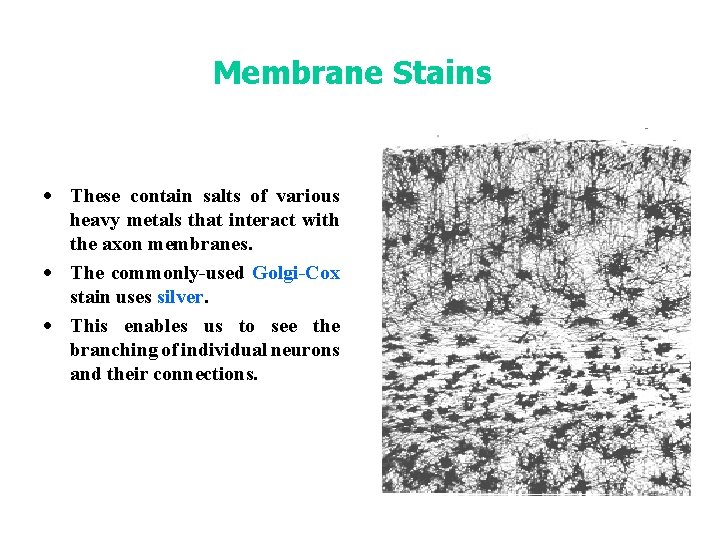
Membrane Stains · These contain salts of various heavy metals that interact with the axon membranes. · The commonly-used Golgi-Cox stain uses silver. · This enables us to see the branching of individual neurons and their connections.

b) Tracing Connections • The structures of the CNS are interconnected by complex systems of axons, finding out what nuclei are connected to what others and the routes taken can be solved by: • i) Anterograde (forward) tracing: Certain proteins are taken up by cell bodies are transported through axons until they reach the terminal buttons. Lectins such as phaseolus vulgaris leukoagglutinin (PHA-L) are often used. • ii) Retrograde (backward) tracing: Dyes such as flurogold are injected into the terminal buttons and are then carried back through the axons to the cell bodies where they can be seen.
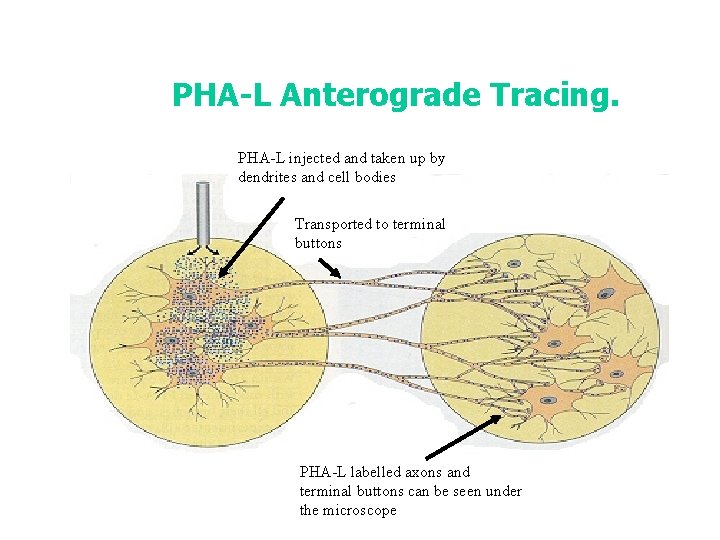
PHA-L Anterograde Tracing. PHA-L injected and taken up by dendrites and cell bodies Transported to terminal buttons PHA-L labelled axons and terminal buttons can be seen under the microscope

c) Histochemical Techniques. • • • These tell us the location of specific neurons that produce and secrete particular neurotransmitters. Antibodies for a specific neurotransmitterare injected into a region and the slides are viewed under ultraviolet light. Alternatively radioactive 2 deoxyglucose is taken up by active neurons. Vasopressin-containing axons and terminal buttons
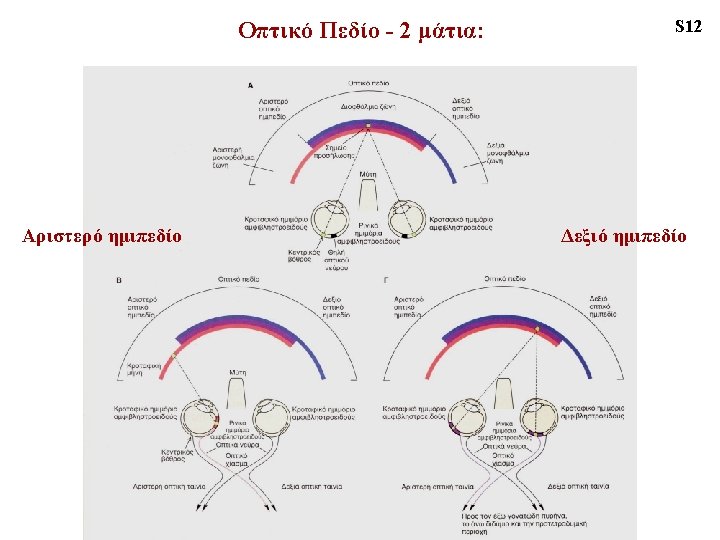
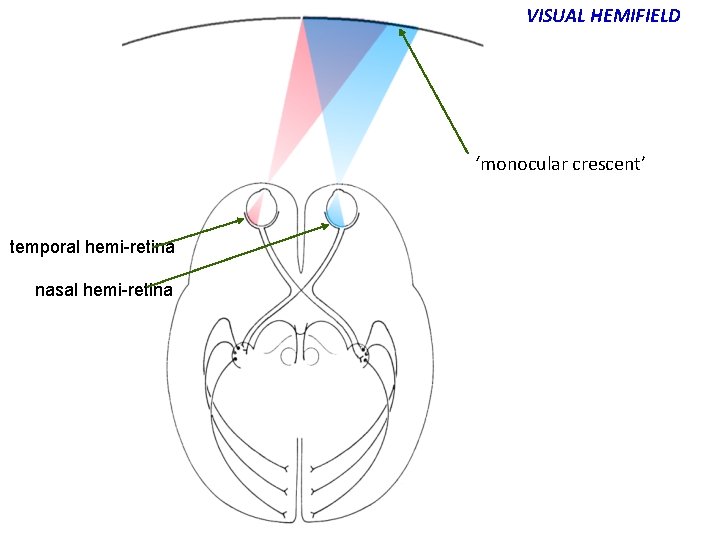
VISUAL HEMIFIELD ‘monocular crescent’ temporal hemi-retina nasal hemi-retina
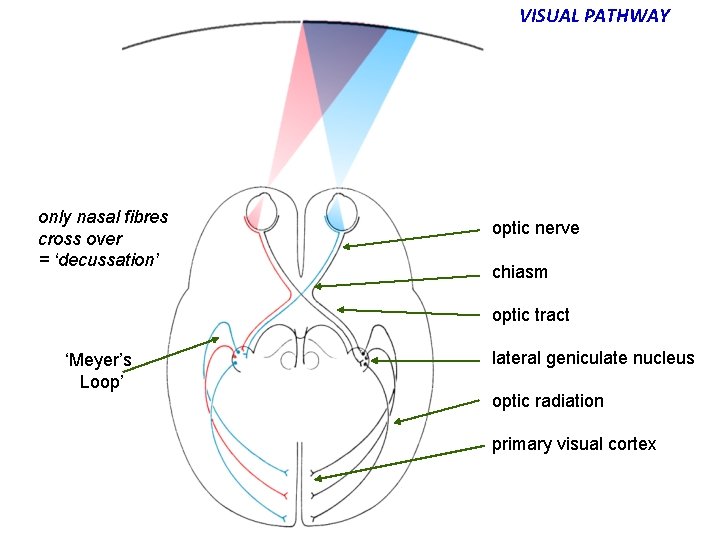
VISUAL PATHWAY only nasal fibres cross over = ‘decussation’ optic nerve chiasm optic tract ‘Meyer’s Loop’ lateral geniculate nucleus optic radiation primary visual cortex
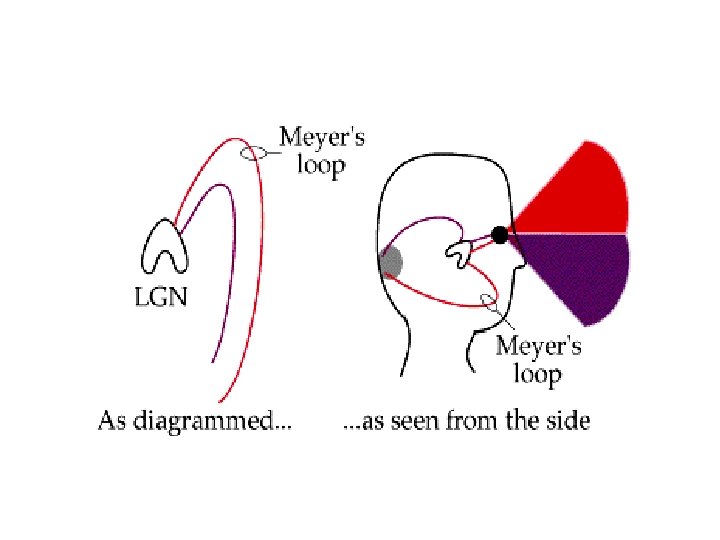
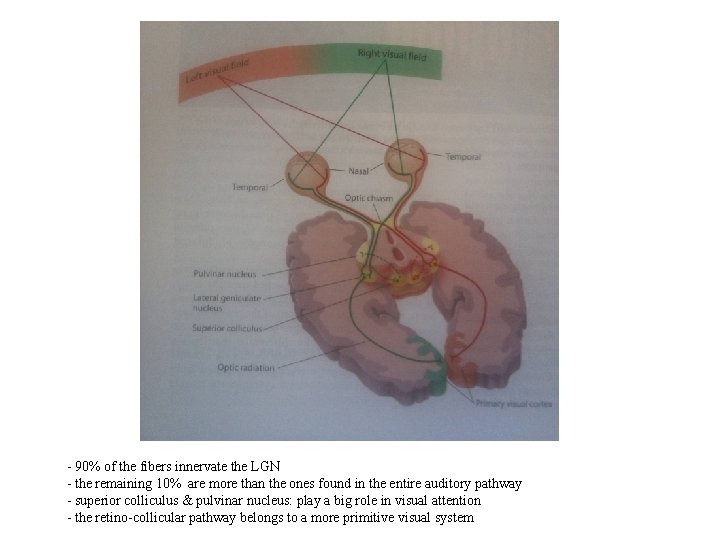
- 90% of the fibers innervate the LGN - the remaining 10% are more than the ones found in the entire auditory pathway - superior colliculus & pulvinar nucleus: play a big role in visual attention - the retino-collicular pathway belongs to a more primitive visual system
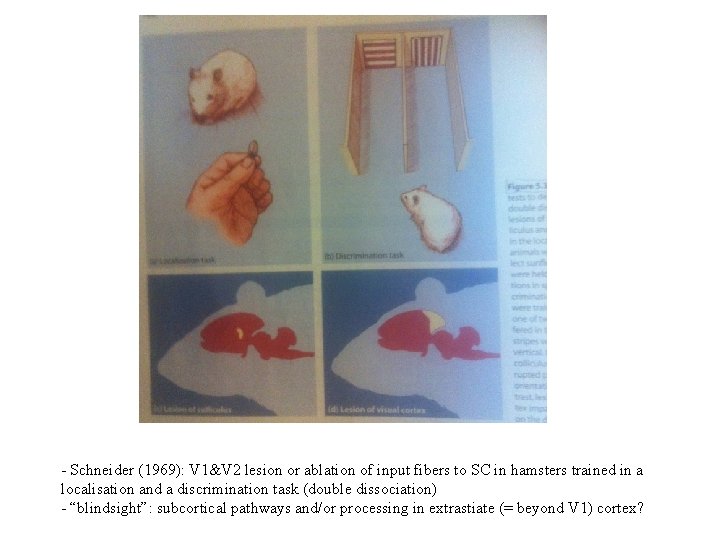
- Schneider (1969): V 1&V 2 lesion or ablation of input fibers to SC in hamsters trained in a localisation and a discrimination task (double dissociation) - “blindsight”: subcortical pathways and/or processing in extrastiate (= beyond V 1) cortex?
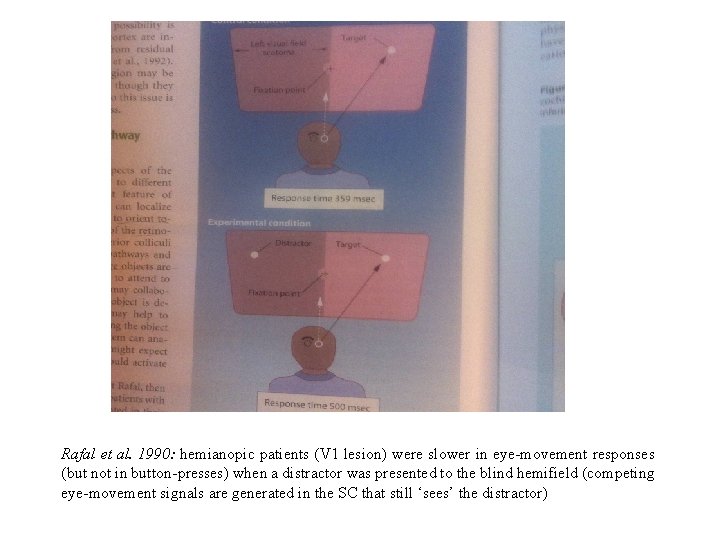
Rafal et al. 1990: hemianopic patients (V 1 lesion) were slower in eye-movement responses (but not in button-presses) when a distractor was presented to the blind hemifield (competing eye-movement signals are generated in the SC that still ‘sees’ the distractor)
- Slides: 14