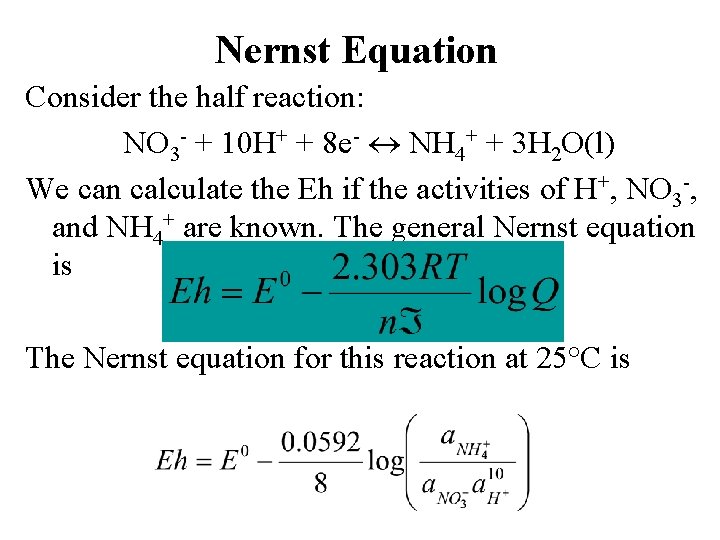
Nernst Equation Consider the half reaction NO 3

- Slides: 15

Nernst Equation Consider the half reaction: NO 3 - + 10 H+ + 8 e- NH 4+ + 3 H 2 O(l) We can calculate the Eh if the activities of H+, NO 3 -, and NH 4+ are known. The general Nernst equation is The Nernst equation for this reaction at 25°C is

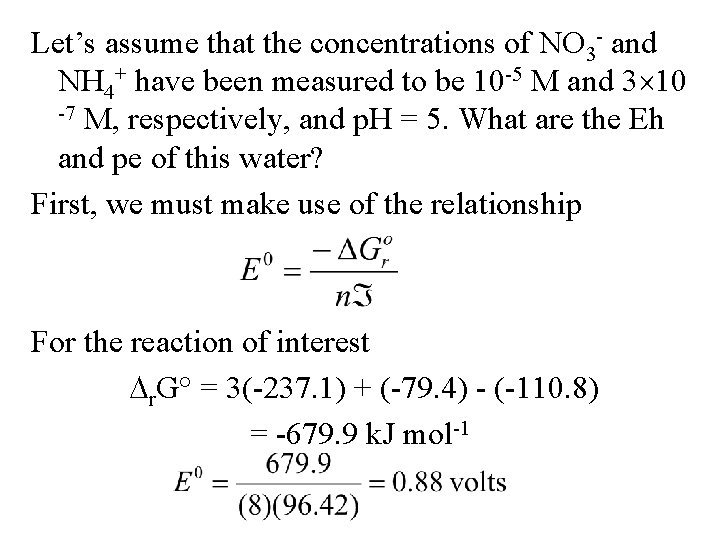
Let’s assume that the concentrations of NO 3 - and NH 4+ have been measured to be 10 -5 M and 3 10 -7 M, respectively, and p. H = 5. What are the Eh and pe of this water? First, we must make use of the relationship For the reaction of interest r. G° = 3(-237. 1) + (-79. 4) - (-110. 8) = -679. 9 k. J mol-1

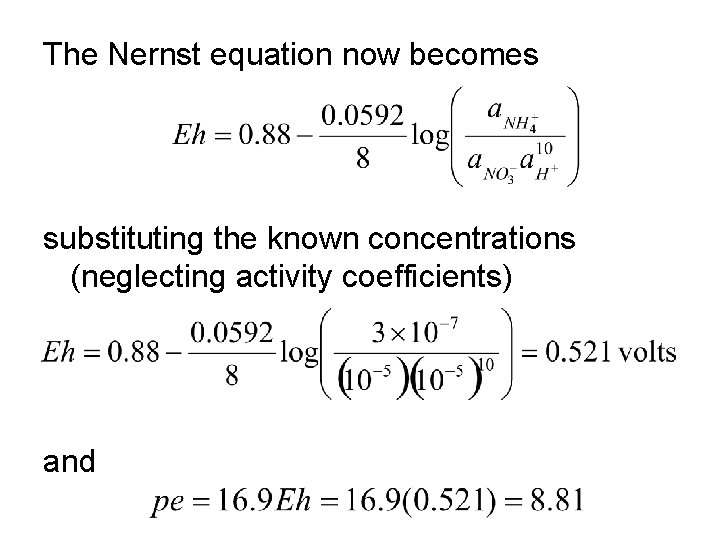
The Nernst equation now becomes substituting the known concentrations (neglecting activity coefficients) and

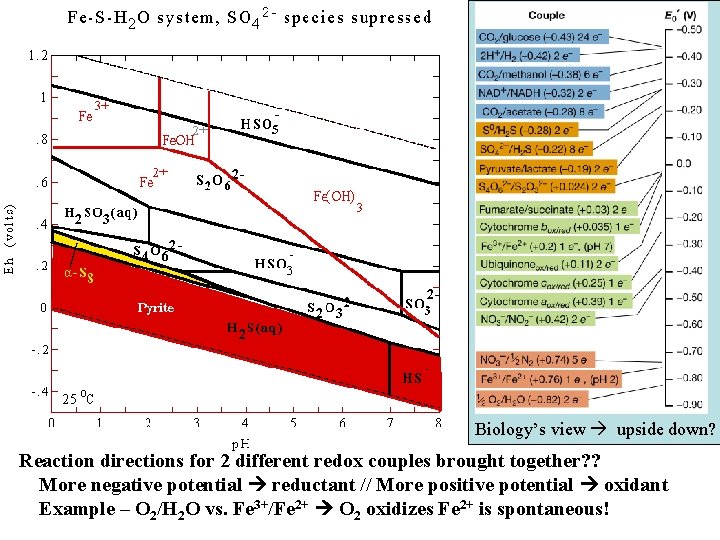
Biology’s view upside down? Reaction directions for 2 different redox couples brought together? ? More negative potential reductant // More positive potential oxidant Example – O 2/H 2 O vs. Fe 3+/Fe 2+ O 2 oxidizes Fe 2+ is spontaneous!

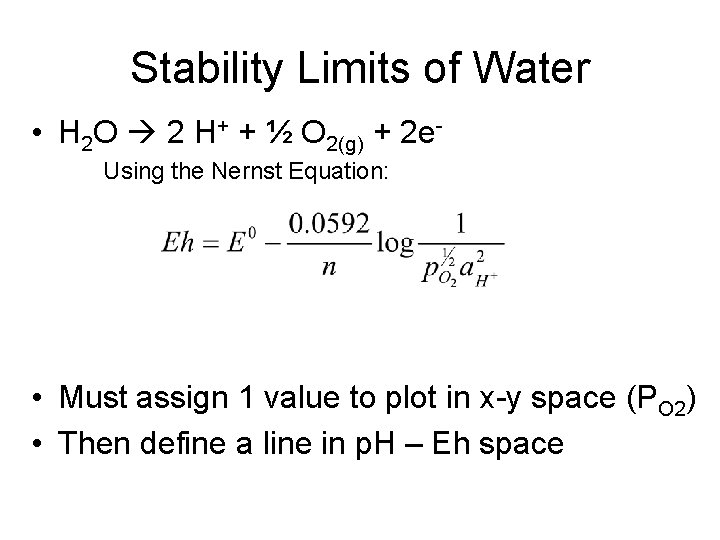
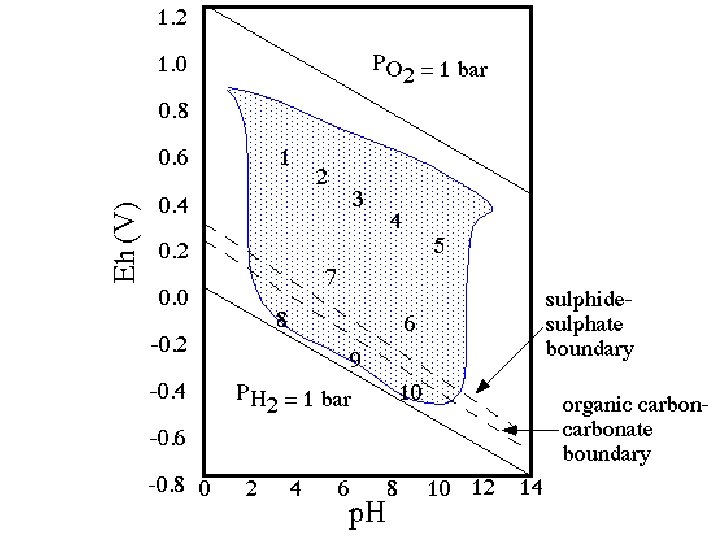
Stability Limits of Water • H 2 O 2 H+ + ½ O 2(g) + 2 e. Using the Nernst Equation: • Must assign 1 value to plot in x-y space (PO 2) • Then define a line in p. H – Eh space

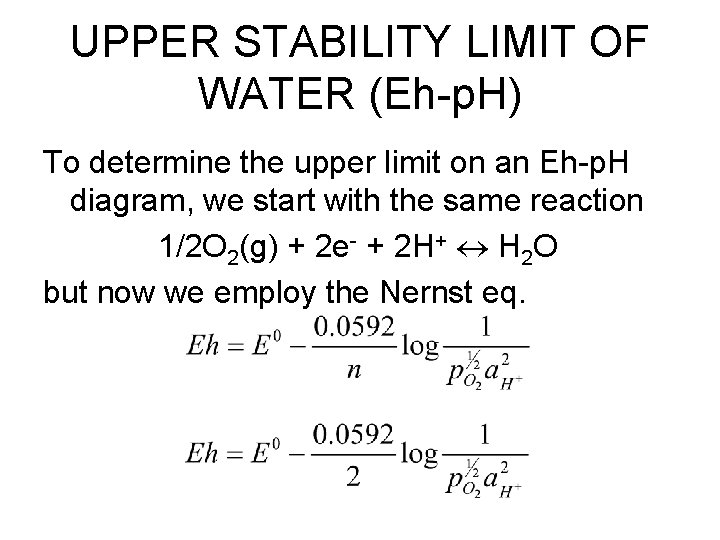
UPPER STABILITY LIMIT OF WATER (Eh-p. H) To determine the upper limit on an Eh-p. H diagram, we start with the same reaction 1/2 O 2(g) + 2 e- + 2 H+ H 2 O but now we employ the Nernst eq.

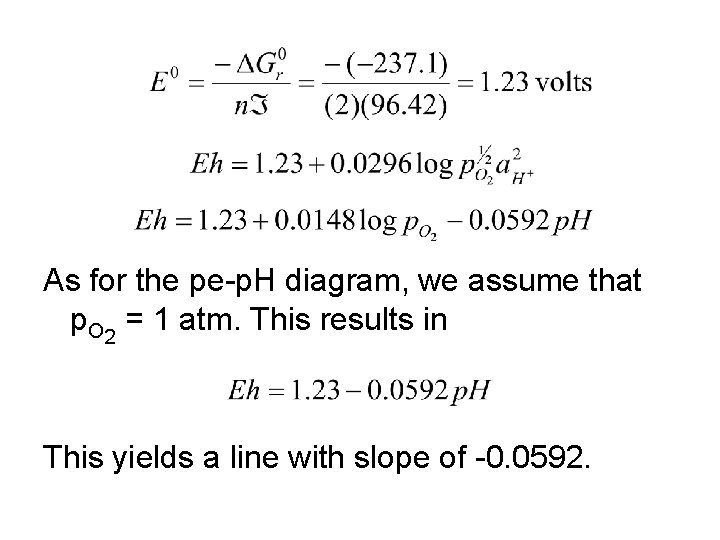
As for the pe-p. H diagram, we assume that p. O 2 = 1 atm. This results in This yields a line with slope of -0. 0592.

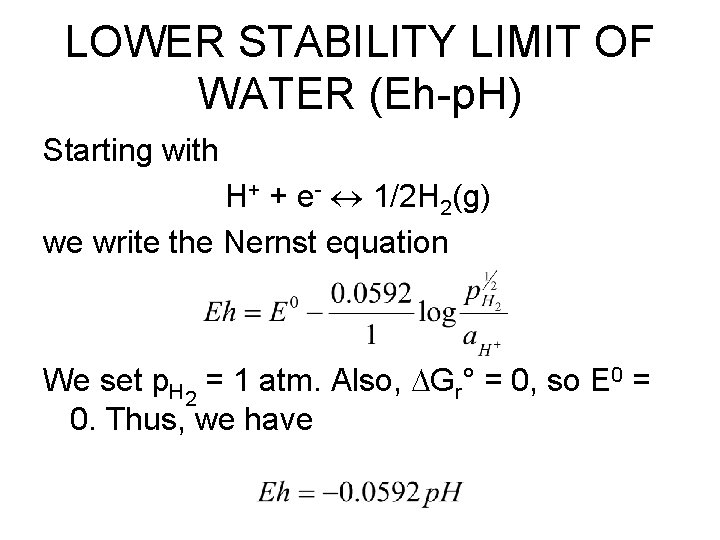
LOWER STABILITY LIMIT OF WATER (Eh-p. H) Starting with H+ + e- 1/2 H 2(g) we write the Nernst equation We set p. H 2 = 1 atm. Also, Gr° = 0, so E 0 = 0. Thus, we have


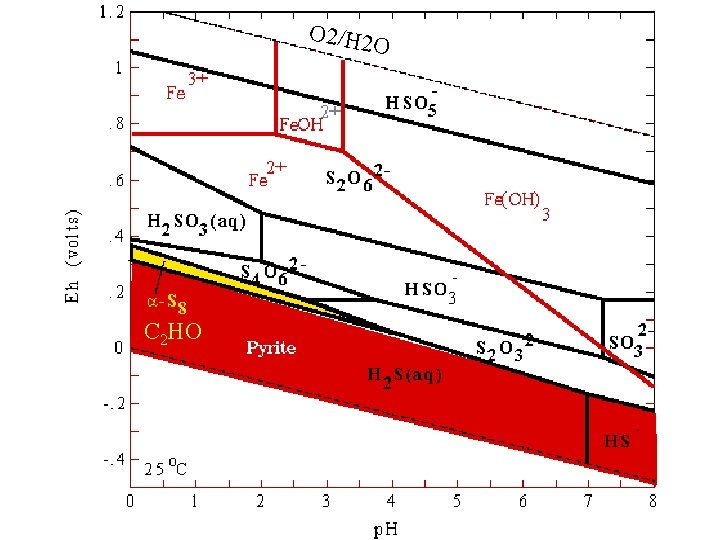
O 2/H 2 O C 2 HO

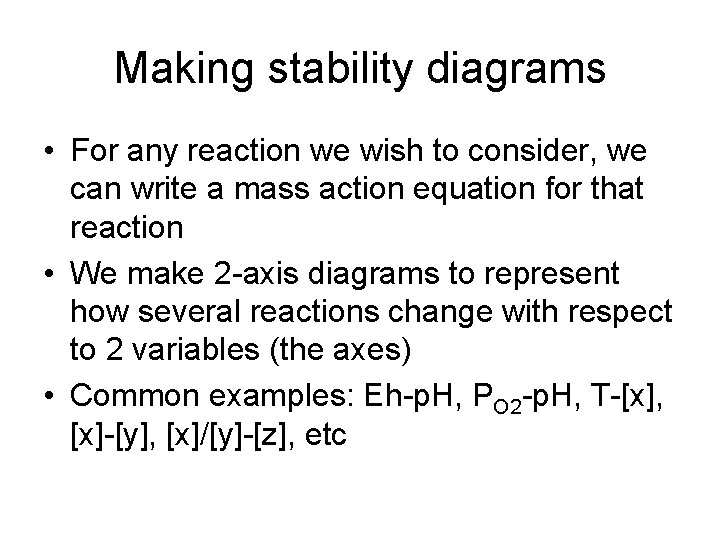
Making stability diagrams • For any reaction we wish to consider, we can write a mass action equation for that reaction • We make 2 -axis diagrams to represent how several reactions change with respect to 2 variables (the axes) • Common examples: Eh-p. H, PO 2 -p. H, T-[x], [x]-[y], [x]/[y]-[z], etc

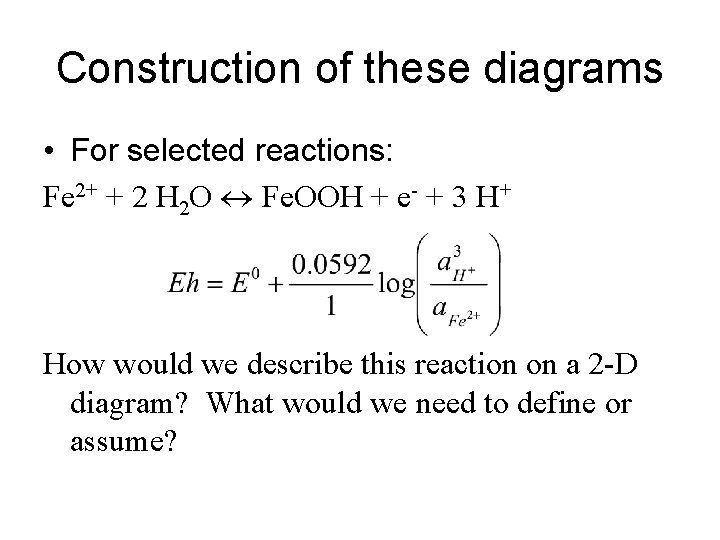
Construction of these diagrams • For selected reactions: Fe 2+ + 2 H 2 O Fe. OOH + e- + 3 H+ How would we describe this reaction on a 2 -D diagram? What would we need to define or assume?

• How about: • Fe 3+ + 2 H 2 O Fe. OOH(ferrihydrite) + 3 H+ Ksp=[H+]3/[Fe 3+] log K=3 p. H – log[Fe 3+] How would one put this on an Eh-p. H diagram, could it go into any other type of diagram (what other factors affect this equilibrium description? ? ? )

Redox titrations • Imagine an oxic water being reduced to become an anoxic water • We can change the Eh of a solution by adding reductant or oxidant just like we can change p. H by adding an acid or base • Just as p. K determined which conjugate acid -base pair would buffer p. H, pe determines what redox pair will buffer Eh (and thus be reduced/oxidized themselves)

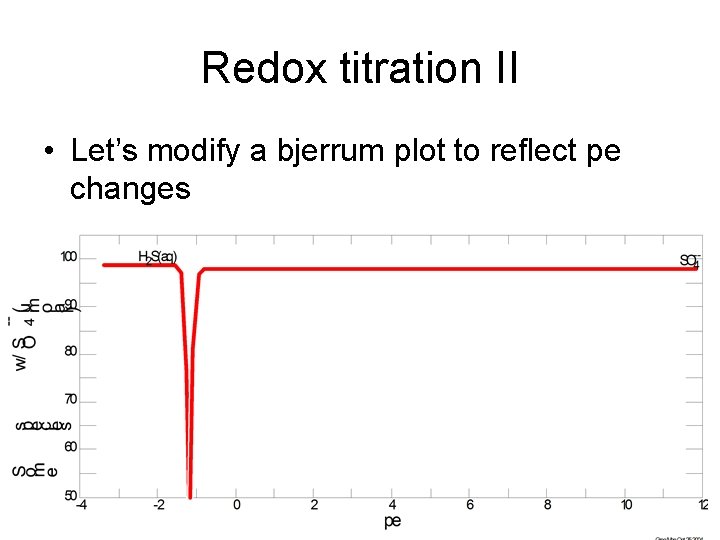
Redox titration II • Let’s modify a bjerrum plot to reflect pe changes