Nephelinesilica and the rest of the basalt tetrahedron

Nepheline-silica and the rest of the basalt tetrahedron Bazalt és fázisdiagramjai doktori kurzus Készítette: Patkó Levente 2016. 02. 09.
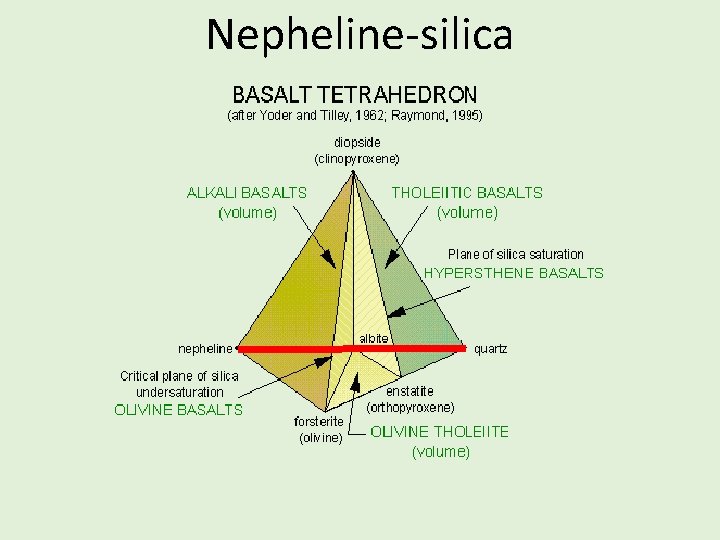
Nepheline-silica

Bevezetés • the appearance of nepheline in the norm is always sufficient evidence of a critically undersaturated rock warranting such names as alkali basalt, basanite, olivine nephelinite, etc. • the natural mineral always contains "excess" silica (deficient alkali and aluminum) • when it is soda rich, the amount of this "excess" decreasing as the composition of Ness varies toward KAISi. O 4 (kalsilite)
![A Ne-Si. O 2 rendszer A nefelin (Na. Al[Si. O 4]) képes szilárd oldatot A Ne-Si. O 2 rendszer A nefelin (Na. Al[Si. O 4]) képes szilárd oldatot](http://slidetodoc.com/presentation_image_h2/50a5a93191d37a7e449477c7dfa70989/image-4.jpg)
A Ne-Si. O 2 rendszer A nefelin (Na. Al[Si. O 4]) képes szilárd oldatot alkotni és ekkor a szerkezetbe Si 2 O épül be. A nefelinnek laboratóriumban előállították a nagy hőmérsékletű változatát, ami a carneigit (Cg) nevet kapta. A Cg 1254 °C és 1526 °C között fordul elő (azért nem hivatalos ásványnév, mert természetes előfordulása nem ismert).
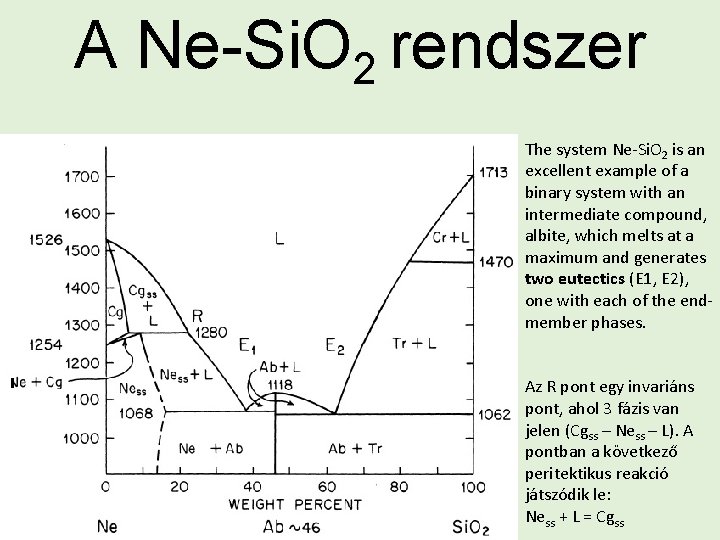
A Ne-Si. O 2 rendszer The system Ne-Si. O 2 is an excellent example of a binary system with an intermediate compound, albite, which melts at a maximum and generates two eutectics (E 1, E 2), one with each of the endmember phases. Az R pont egy invariáns pont, ahol 3 fázis van jelen (Cgss – Ness – L). A pontban a következő peritektikus reakció játszódik le: Ness + L = Cgss
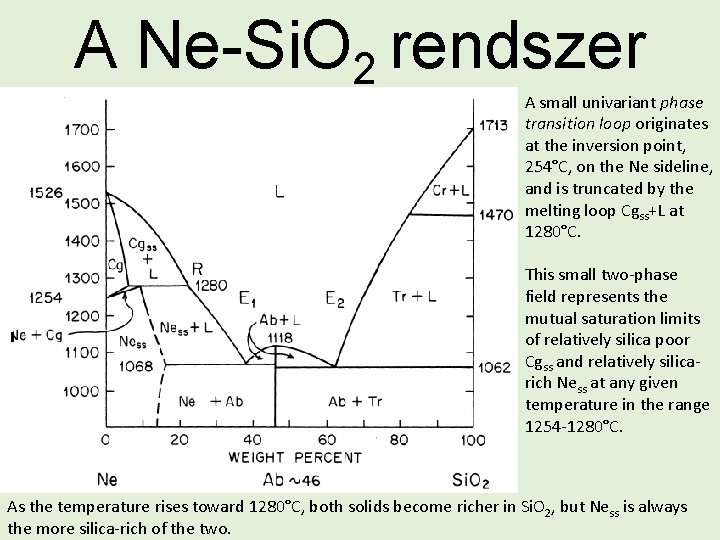
A Ne-Si. O 2 rendszer A small univariant phase transition loop originates at the inversion point, 254°C, on the Ne sideline, and is truncated by the melting loop Cgss+L at 1280°C. This small two-phase field represents the mutual saturation limits of relatively silica poor Cgss and relatively silicarich Ness at any given temperature in the range 1254 -1280°C. As the temperature rises toward 1280°C, both solids become richer in Si. O 2, but Ness is always the more silica-rich of the two.
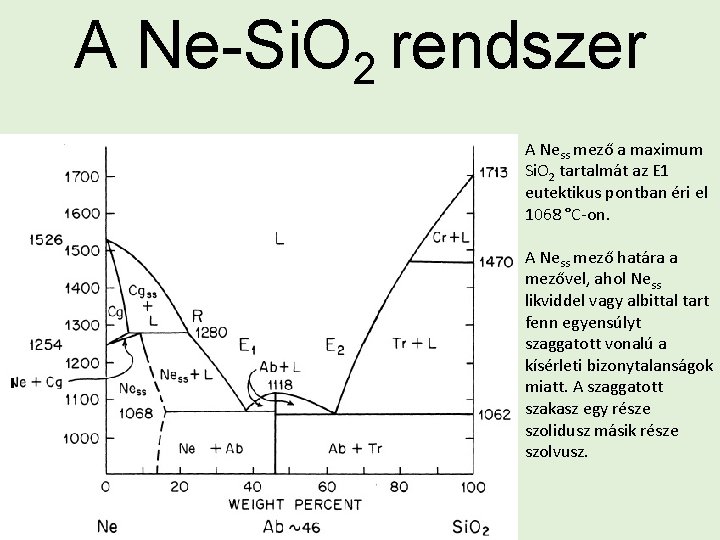
A Ne-Si. O 2 rendszer A Ness mező a maximum Si. O 2 tartalmát az E 1 eutektikus pontban éri el 1068 °C-on. A Ness mező határa a mezővel, ahol Ness likviddel vagy albittal tart fenn egyensúlyt szaggatott vonalú a kísérleti bizonytalanságok miatt. A szaggatott szakasz egy része szolidusz másik része szolvusz.
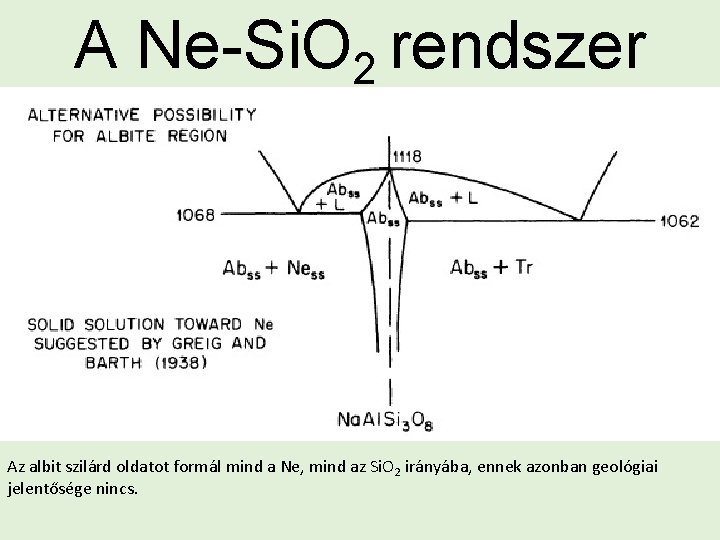
A Ne-Si. O 2 rendszer Az albit szilárd oldatot formál mind a Ne, mind az Si. O 2 irányába, ennek azonban geológiai jelentősége nincs.
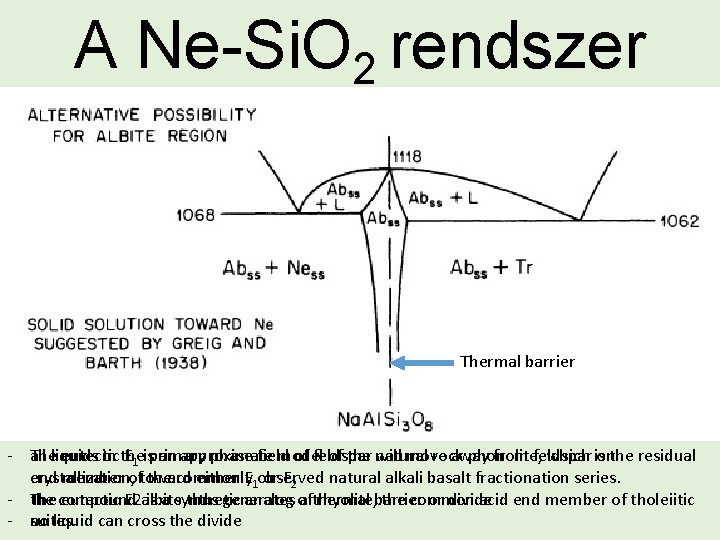
A Ne-Si. O 2 rendszer Thermal barrier - The all liquids eutectic in the E 1 isprimary an approximate phase field model of feldspar of the natural will move rock away phonolite, from feldspar which is onthe residual end crystallization, member oftoward the commonly either E 1 observed or E 2 natural alkali basalt fractionation series. - The the compound eutectic E 2 albite is a synthetic thus generates analog of a thermal rhyolite, barrier the common or divide acid end member of tholeiitic - suites. no liquid can cross the divide
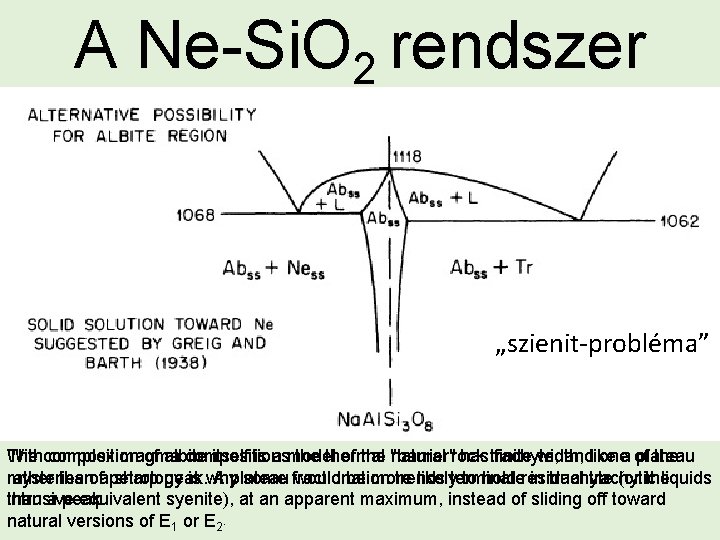
A Ne-Si. O 2 rendszer „szienit-probléma” The Withcomposition complex magma of albite compositions itself is a model thermal of the "barrier" natural rock has trachyte, finite width, andlike onea of plateau the mysteries rather thanofapetrology sharp peak. is why A plateau some fractionation would be more trends likelyterminate to hold residual in trachyte trachytic (or theliquids intrusive than a peak. equivalent syenite), at an apparent maximum, instead of sliding off toward natural versions of E 1 or E 2 •
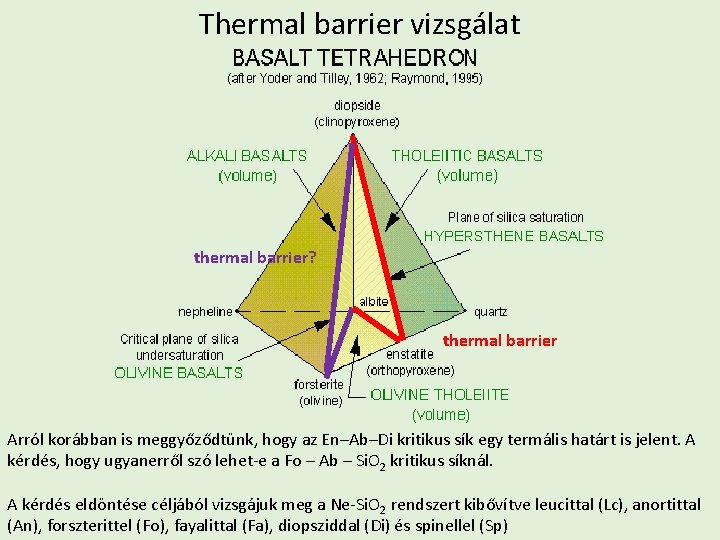
Thermal barrier vizsgálat thermal barrier? thermal barrier Arról korábban is meggyőződtünk, hogy az En–Ab–Di kritikus sík egy termális határt is jelent. A kérdés, hogy ugyanerről szó lehet-e a Fo – Ab – Si. O 2 kritikus síknál. A kérdés eldöntése céljából vizsgájuk meg a Ne-Si. O 2 rendszert kibővítve leucittal (Lc), anortittal (An), forszterittel (Fo), fayalittal (Fa), diopsziddal (Di) és spinellel (Sp)
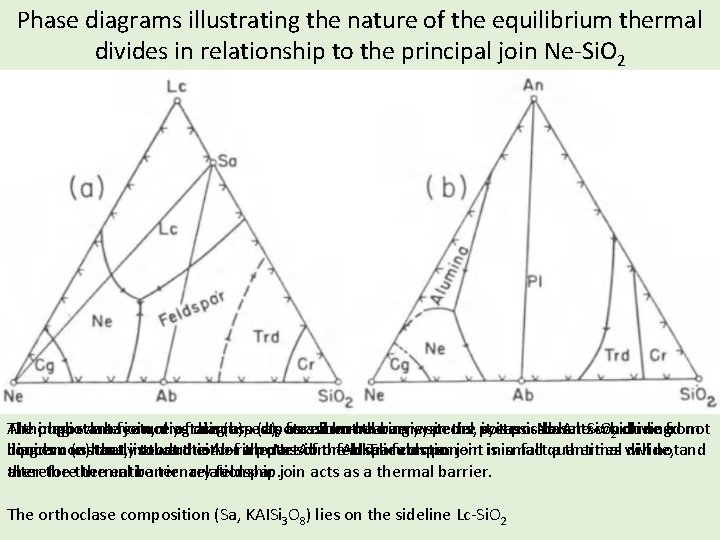
Phase diagrams illustrating the nature of the equilibrium thermal divides in relationship to the principal join Ne-Si. O 2 Although we have notdiagram yet discussed it is possible to conclude from The plagioclase important feature join, of diagram (b), acts (a), potassium-bearing for as aall thermal but thebarrier verysystems, special in the potassic system Ne-An-Si. O basalts which need not 2 driving diagram (a)here, that introduction of athe potassium component inin small notand liquids constantly concern us istoward that the either Ab-rich part Ne-Ab of the orfeldspar Ab-Tr alkalieutectic. feldspar join is factquantities a thermal will divide, alter thermal barrier relationship. therefore the entire ternary feldspar join acts as a thermal barrier. The orthoclase composition (Sa, KAISi 3 O 8) lies on the sideline Lc-Si. O 2
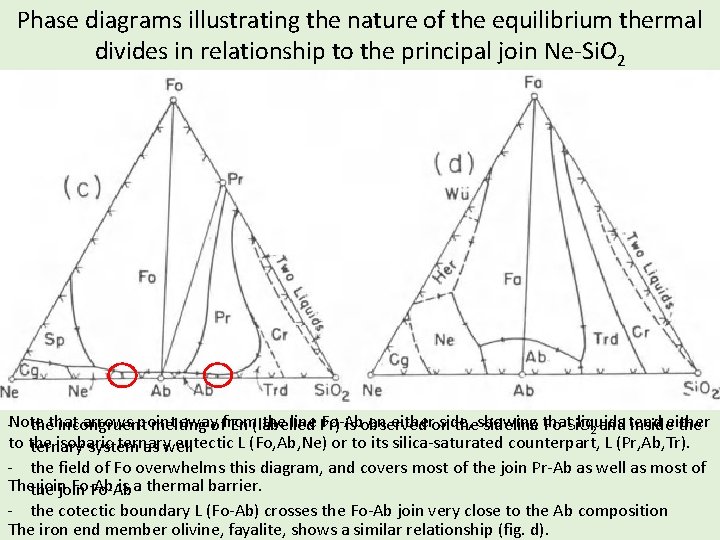
Phase diagrams illustrating the nature of the equilibrium thermal divides in relationship to the principal join Ne-Si. O 2 arrows point awayoffrom the line Pr) Fo-Ab on eitheron side, liquids tend either -Note thethat incongruent melting En (labelled is observed theshowing sideline that Fo-Si. O 2 and inside the to the isobaric ternary eutectic L (Fo, Ab, Ne) or to its silica-saturated counterpart, L (Pr, Ab, Tr). ternary system as well - the field of Fo overwhelms this diagram, and covers most of the join Pr-Ab as well as most of Thethe join Fo-Ab is a thermal barrier. Fo-Ab - the cotectic boundary L (Fo-Ab) crosses the Fo-Ab join very close to the Ab composition The iron end member olivine, fayalite, shows a similar relationship (fig. d).
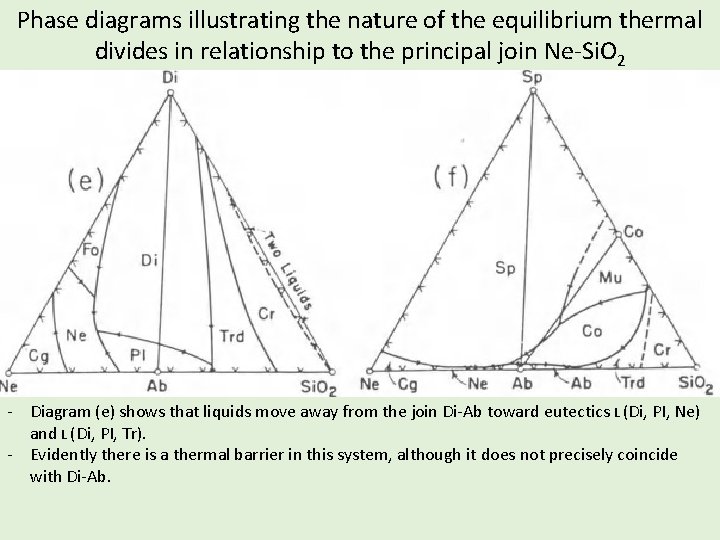
Phase diagrams illustrating the nature of the equilibrium thermal divides in relationship to the principal join Ne-Si. O 2 - Diagram (e) shows that liquids move away from the join Di-Ab toward eutectics L (Di, PI, Ne) and L (Di, PI, Tr). - Evidently there is a thermal barrier in this system, although it does not precisely coincide with Di-Ab.
Thermal barrier vizsgálat We may therefore conclude from the diagrams that the plagioclase feldspars (including varieties as K-rich as anorthoclase, or calcic sanidine) constitute a thermal divide between merely undersaturated and critically undersaturated liquids, that this divide lies very near the critical plane in the basalt tetrahedron, and that the properties of the divide persist in the presence of iron-bearing silicates such as fayalite. Kivétel, amikor a barrier átjárható: This mechanism is oxidation or reduction of iron in the magma. The effect of oxidation on silica saturation may be simply stated, however, by noting that if the ferrous iron normally incorporated in silicates (e. g. , fayalite) is oxidized enough to cause formation of magnetite (Fe. O· Fe 2 O 3) , the crystallization of this non-silicate mineral will "release" silica to the residual liquid which otherwise would have been bound up in ferrous silicates. Apart from the effects of pressure and oxidation-reduction reactions, the concept of thermal barrier appears to be a durable one. We may therefore state that at low pressures and within certain ranges of oxygen partial pressures, no normal basaltic liquid can make a transit of the natural critical plane of silica undersaturation; alkali basalts cannot be parental to olivine tholeiites, and vice versa.
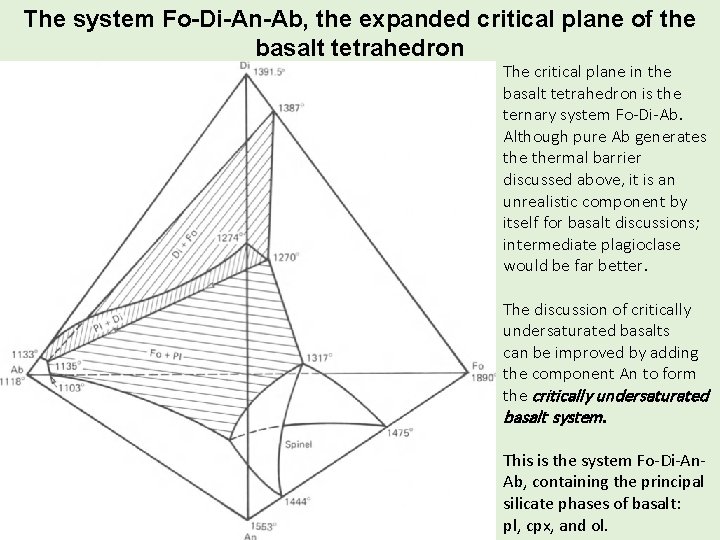
The system Fo-Di-An-Ab, the expanded critical plane of the basalt tetrahedron The critical plane in the basalt tetrahedron is the ternary system Fo-Di-Ab. Although pure Ab generates thermal barrier discussed above, it is an unrealistic component by itself for basalt discussions; intermediate plagioclase would be far better. The discussion of critically undersaturated basalts can be improved by adding the component An to form the critically undersaturated basalt system. This is the system Fo-Di-An. Ab, containing the principal silicate phases of basalt: pl, cpx, and ol.
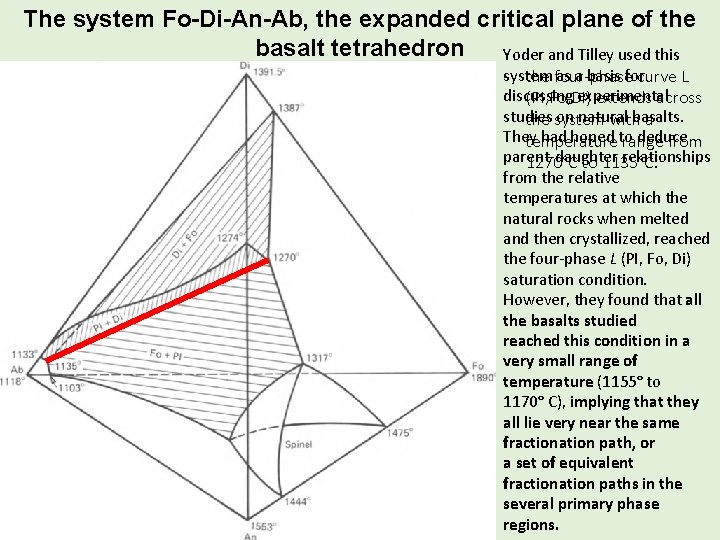
The system Fo-Di-An-Ab, the expanded critical plane of the basalt tetrahedron Yoder and Tilley used this as a basis forcurve L -system the four-phase discussing experimental (PI, Fo, Di) extends across studies on natural basalts. the system with a They had hoped to deduce temperature range from parent-daughter relationships 1270°C to 1135°C. from the relative temperatures at which the natural rocks when melted and then crystallized, reached the four-phase L (PI, Fo, Di) saturation condition. However, they found that all the basalts studied reached this condition in a very small range of temperature (1155° to 1170° C), implying that they all lie very near the same fractionation path, or a set of equivalent fractionation paths in the several primary phase regions.

Felmerülő bazalt nevezéktani megjegyzés • Pikrites bazalt: az a speciális eset, amikor a gyorsan felszín felé hatoló magmában gyakorlatilag nem zajlik frakcionációs kristályosodás útja során, ami az ultramafikus összetétel megtartását eredményezi. • Afíros bazalt: aprószemcsés bazalt, ahol vagy nem történt a bazalt útja során frakcionációs kristályosodás, vagy a frakcionálódó termékek visszamaradtak.

Fontos megállapítások • The critical plane divides the tetrahedron into three fundamentally different regions, the undersaturated basalts, which fractionate away from the plane and toward Ne, the critically undersaturated basalts which always remain critically undersaturated in the trachyte trend, and the tholeiitic basalts which fractionate away from the plane and toward hypersthene or silica saturation. • Alkali basalt cannot, at low pressures, fractionate to produce tholeiitic basalt (unless oxidation is involved), nor can tholeiitic basalt fractionate to produce alkali basalt. • Within the tholeiitic volume to the silica-rich side of the critical plane, olivine tholeiites may fractionate to produce hypersthene basalt or quartz basalt; the reverse process cannot occur. Olivine tholeiites can be and no doubt often are parental to any of the suite of rocks having higher degrees of silica saturation, up to and including rhyolite (in small amounts controlled by the amount of alkalies, Na 2 O and K 2 O, in the initial magma).

Forrás Az összes ábra forrása a Morse-könyv. Morse, S. A. (1980). Basalts and phase diagrams: an introduction to the quantitative use of phase diagrams in igneous petrology. Springer Verlag
- Slides: 20