Neonatal sepsis Sepsis neonatarum Risk factors for neonatal


























- Slides: 39

Neonatal sepsis (Sepsis neonatarum)

Risk factors for neonatal sepsis: 1. Vaginal colonization with group B streptococcus. 2. Prolonged rupture of membranes (>24 hr). 3. Amnionitis. 4. Maternal fever or leukocytosis. 5. Fetal tachycardia. 6. Preterm delivery. 7. Black race and male sex. 8. Congenital immune deficiency or asplenia. 9. Presence of galactosemia (E. coli sepsis). 10. Congenital malformation e. g. obstructive uropathy.

Portal of entery : acental hematogenous spread al transmission) of M. O. from the to her infant which might occur at nt times during with manifestation of present at birth or delayed for months or years. nding infections through the or without rupture of nes, which might results in mnionitis, funisitis (infection of umbilical cord), congenital pneumonia or sepsis.

3. Infection acquired from passage through an cted birth canal or exposure to infected ood at delivery (e. g. herpes simplex, hepatitis B, bacterial infection) Nosocomial infection which occurs after birth e to neonatal exposure to various infectious in the nursery or in the community, ly in the presence of central vein ilical vessels catheter, acheal tube, or electronic monitoring devices.

Types of neonatal sepsis: It's of 3 types according to the time of onset which are early-onset, late-onset and nosocomial sepsis. I. Early-onset sepsis: Its manifested at period from birth-7 days of life, the infection is usually begins in utero and usually is due to infection by bacteria in the mother GUT, which include group B streptococcus, E. coli, Klebsiella, L. monocytogenes, and nontypable H. influenzae.

II. Late-onset neonatal sepsis: In which the manifestations begin between 8 -28 days of life, its usually occur in healthy full-term neonate who was discharged in good health from the normal newborn nursery. Causative microorganisms of late-onset sepsis are similar to those in early-onset sepsis, but lateonset sepsis may be caused by the pathogens usually found in older infants (H. influnzae type B, streptococcus pneumoniae, N. meningitides), in addition to viral agents (HSV, CMV).

III. Nosocomially acquired sepsis: (8 days-discharge) Occurs predominantly in premature infants in the NICU, many of these infants have been infected with multidrug-resistant bacteria which present in NICU Risk factors: ent treatment with broad spectrum antibiotics for sepsis in the NICU. e of central venous catheters, dotracheal tubes, umbilical vessel catheters, and electronic monitoring devices.

Causative microorganisms: Commonly s. aureus (occasionally methicillinresistant), S. epidermidis (usually methicillinresistant), and gram negative pathogens.

Clinical features: ly manifestations include grunting, poor eding, pallor, apnea, lethargy, hypothermia or (which is non specific manifestations). eterm infants, it's often very difficult to ntiate sepsis from RDS, so that most nfants with RDS receive broad spectrum antibiotics. organ system disease manifested by ratory failure, shock, DIC, acute tubular necrosis and symmetric peripheral gangrene.

4. Hematogenous spread (especially in late-onset sepsis): may result in focal infection such as meningitis (75%of late-onset and 30% of early-onset sepsis cases), osteomylitis (group B streptococci, s. aureus), arthritis (gonococcus, s. aureus, Candida albicans), and UTI (gram negative bacteria). comial sepsis might associated with mphalitis, eye discharge, diarrhea and bullous impetigo.

Investigations: 1. Blood culture and urine culture. Cerebrospinal fluid (CSF) for gram stain, cells count, protein and culture. Normal newborn infants have elevated CSF protein (100 -150 mg/dl), and as many as 25 WBC, 75% of which are lymphocytes in the absence of infection of bacterial and viral antigens in mmunoelectrophoresis or Latex agglutination test.

BC to identify neutropenia, an ased numbers of immature neutrophils (bands) and thrombocytopenia. 5. C-reactive protein levels which are elevated in bacterial sepsis. 6. Chest X-ray to detect pneumonia. rial blood gases to detect hypoxia and metabolic acidosis. od pressure, urine output and peripheral fusion monitoring to determine the need to t septic shock with fluids and vasopressor agents (dopamine, dobutamine).

Treatment: Combination of ampicilline and gentamycin for -14 days is effective against most organisms responsible for early-onset sepsis. If meningitis is present, the treatment is extended to 21 days, or 14 days after a negative CSF culture. **If gram negative meningitis present or increase resistance rate to ampicillin, as in lateonset sepsis, most centers change to a third generation cephalosporines (cefotaxime or ceftazidine) plus ampicillin.

**Because the nosocomial sepsis is caused by multidrug-resistance bacteria which present in the NICU, so it treated by combination of Vancomycin or naficillin with gentamycin. **The dose and intervals of all aminoglycosides, such as gentamycin, vary with postnatal age and birth weight. Treatment with aminoglycoside for more than 3 days needs monitoring of serum levels to avoid ototoxicity and nephrotoxicity. Persistent signs of infection despite antibiotics treatment should suggest candidal or viral sepsis.

Inhaled nitric oxide and/or ECMO for treatment of septic-related pulmonary hypertension. Treatment of septic shock by I. V. fluid, plasma expanders, and vasopressor agents.

Congenital infections (STORCH)

Congenital syphilis: Its caused by spirochete Treponema pallidum. Newborn infants acquired infection transplacentally from an infected mother to her fetus and, less commonly, at birth, in postpartum period, or by infected blood.

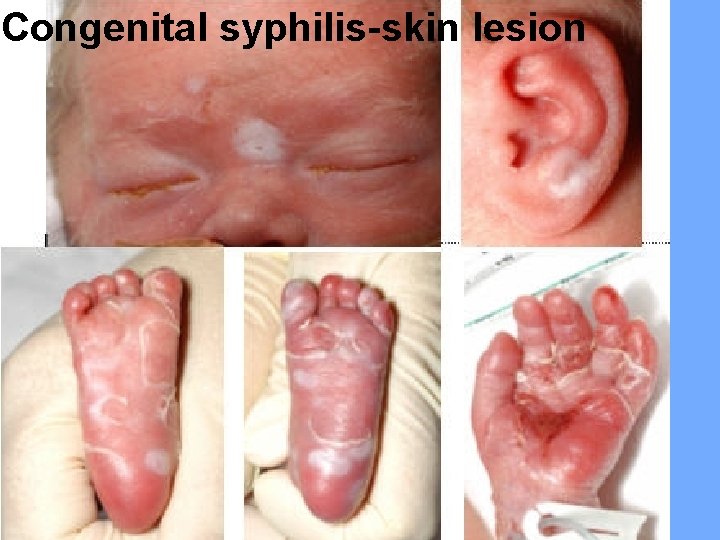
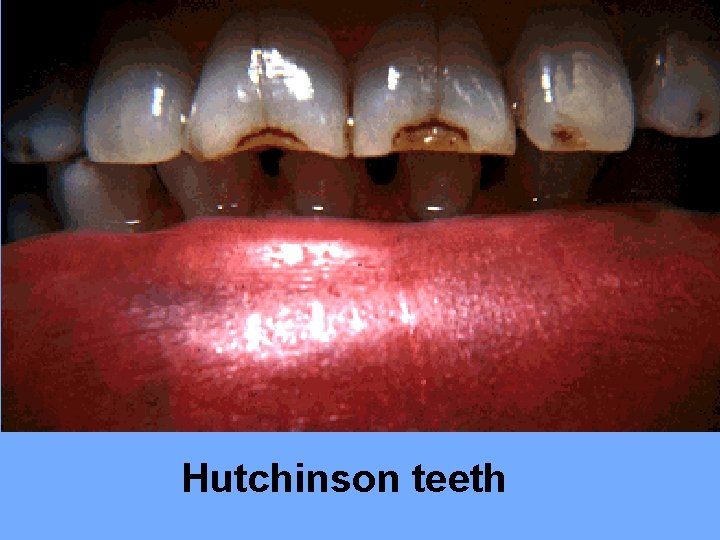
Clinical features: Early manifestations: occurring between birth and 1 st year of life, including: Fever, anemia, failure to thrive, irritability, mucocutaneous lesions (maculopapular rash on trunk, palms and soles, condylomata, bullous eruption), persistent rhinitis (snuffles), HSM, lymphadenopathy, and pseudoparalysis due to osteochondritis. Late manifestations: appears many years after birth and manifested as multiple bone signs (frontal bossing, saber shins), Hutchinson teeth (screw-like teeth), and saddle nose deformity.

Congenital syphilis-skin lesion

Snuffles saber shins

Hutchinson teeth

Saddle nose

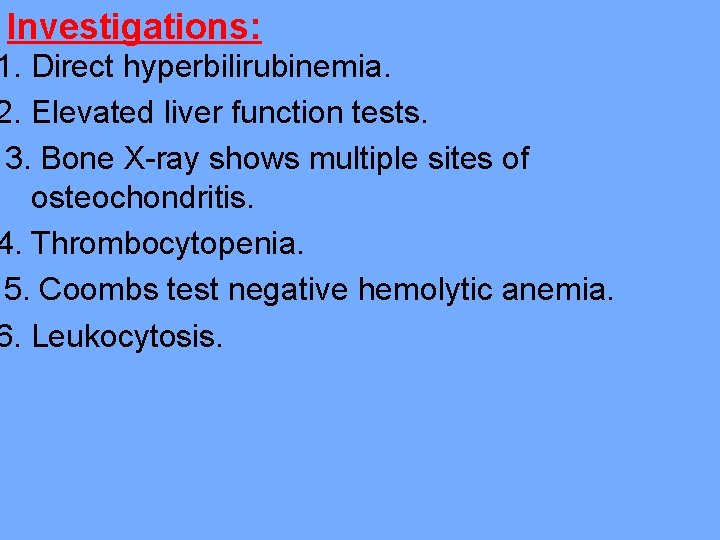
Investigations: 1. Direct hyperbilirubinemia. 2. Elevated liver function tests. 3. Bone X-ray shows multiple sites of osteochondritis. 4. Thrombocytopenia. 5. Coombs test negative hemolytic anemia. 6. Leukocytosis.

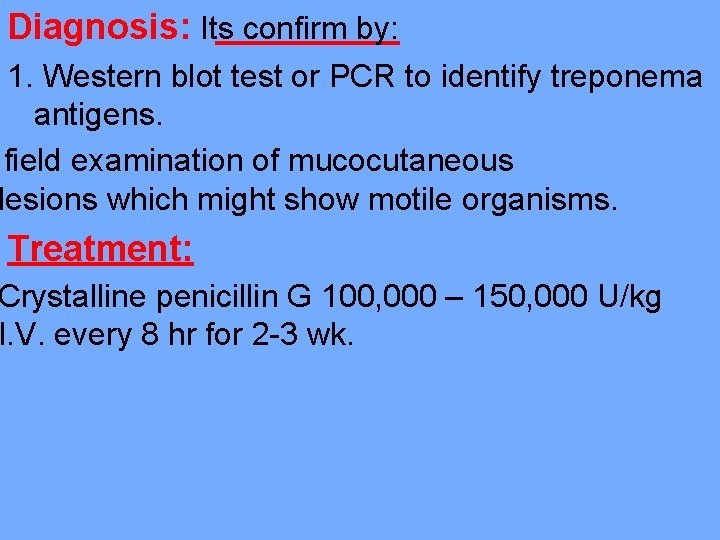
Diagnosis: Its confirm by: 1. Western blot test or PCR to identify treponema antigens. field examination of mucocutaneous lesions which might show motile organisms. Treatment: Crystalline penicillin G 100, 000 – 150, 000 U/kg I. V. every 8 hr for 2 -3 wk.

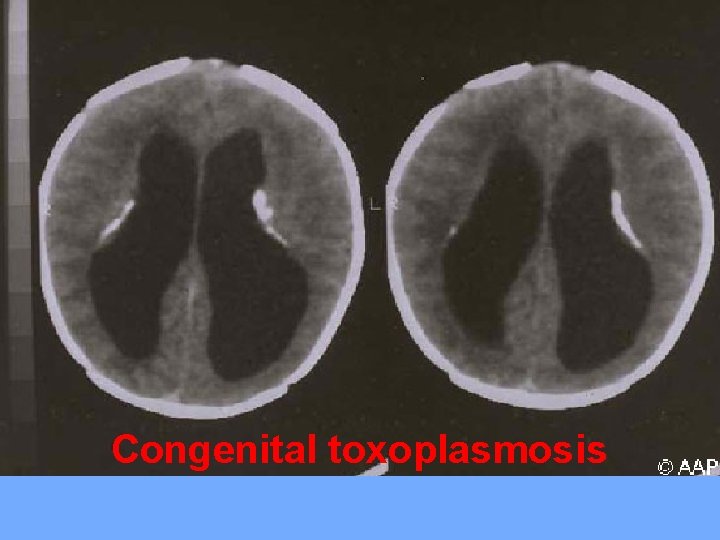
Congenital toxoplasmosis: It's caused by Toxoplasma gondii parasite, and congenital infection occur through transplacental transmission during acute infection of pregnant women.

Clinical features: Most infected mothers are asymptomatic, and if infection occur during pregnancy, 40 -60% of infants will be infected. If mother infected latter in the pregnancy (3 rd trimester), the fetus will be more likely to be infected, but with less sever illness than that if the infection occurs earlier in pregnancy. Severely infected fetus will be stillborn. Live infants have poor feeding, fever, rash, petichae, LAP, HSM, jaundice, hydrocephalus or microcephaly, microphthalmia, seizures, cerebral calcification and chorioretinitis.

Congenital toxoplasmosis

Congenital toxoplasmosis

Diagnosis: By detection of Ig. M anti-toxoplasma antibodies. Treatment: combination of oral pyrimethamine and sulfadiazine for 1 year. Doses: Pyrimethamine 1 -2 mg/kg/24 hr for 2 days then 1 mg/kg/24 hr for 2 mo or 6 mo, then 1 mg/kg/24 hr 3 days a week. Sulfadiazine 100 mg/kg/24 hr loading dose, then 10 mg/kg/24 hr in 2 divided doses

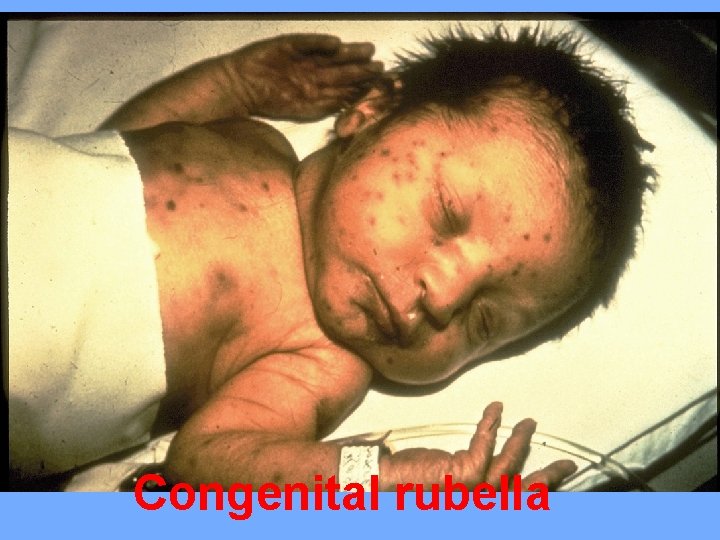
Congenital rubella syndrome: Its serious multisystemic syndrome, acquired from infected mother with rubella virus, characterized by wide spectrum of clinical manifestations and congenital defects. The risk of congenital defects and disease is greater with primary maternal infection during the first trimester, congenital defects occur in about 90% of infants if mother acquired infection during 1 st trimester.

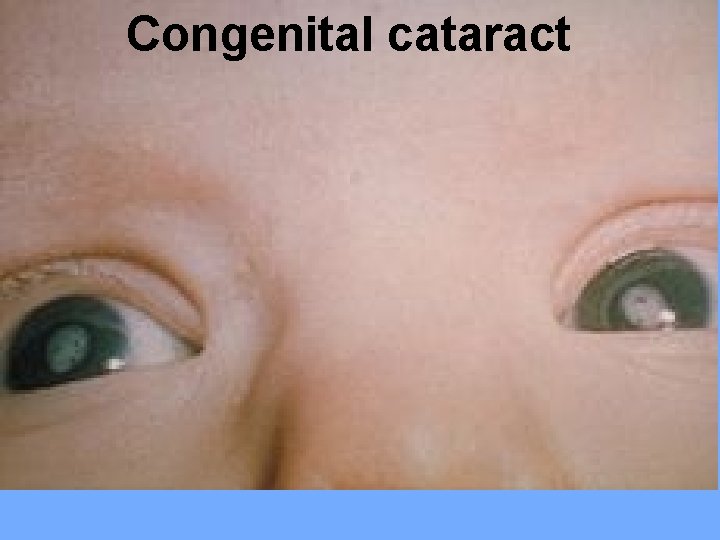
Clinical features: IUGR (most common), cataract, microphthalmia, myocarditis, and congenital heart diseases (PDA, or pulmonary artery stenosis), sensorineural deafness and meningoencephalitis. Persistent infection leads to pneumonia, hepatitis, thrombocytopenia, and anemia. Later sequale include motor and mental retardation.

Congenital rubella

Congenital cataract

Diagnosis: Is confirmed by finding rubella-specific Ig. M antibodies in the neonatal serum or by culturing rubella virus from infant (nosopharynx, urine or tissues). Virus can be shed in the urine for 1 yr or longer.

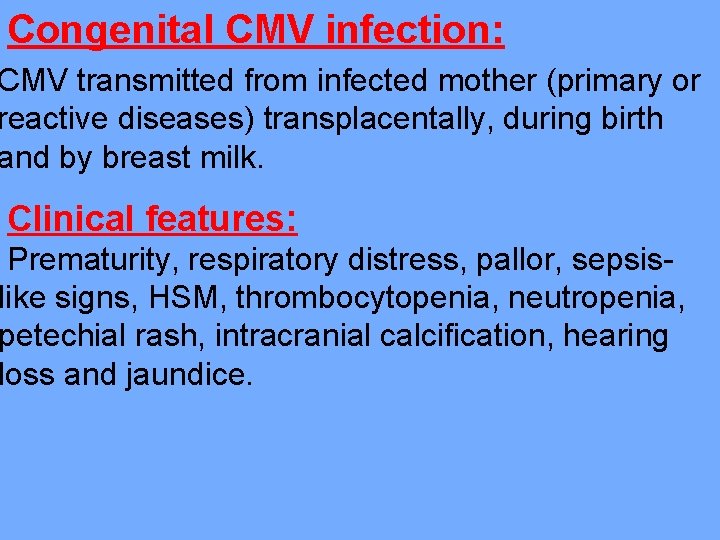
Congenital CMV infection: CMV transmitted from infected mother (primary or reactive diseases) transplacentally, during birth and by breast milk. Clinical features: Prematurity, respiratory distress, pallor, sepsislike signs, HSM, thrombocytopenia, neutropenia, petechial rash, intracranial calcification, hearing loss and jaundice.

Diagnosis: ection of CMV in the urine, pharyngeal ns or peripheral blood leukocytes during 1 st week of life. 2. Serological tests to detect a positive CMV-Ig. M antibodies. Treatment: by antiviral drugs (acyclovir or fascarent).

Neonatal herpes simplex virus (HSV) infection: Majority of cases of neonatal HSV infections are caused by HSV-2 and are acquired when the infant pass through an infected birth canal. Most infected mothers are asymptomatic, and usually no history of maternal or paternal genital HSV infection.

Clinical features: Its caused disseminated (systemic) neonatal infection with initial symptoms occurs within 1 week of birth, these manifestations are: 1. Vesicular or pustular rash 2. Nonfocal meningoencephalitis syndrome. 3. Respiratory distress syndrome. alized systemic infection involving several organs including CNS and liver. emic infection progresses to acidosis, gulopathy, shock, and death in majority of treated cases.

Diagnosis: By PCR or tissue culture for virus. Treatment: by I. V. acyclovir.