NEON TUBES Biogeochemical Cycles What are Biogeochemical Cycles

NEON TUBES Biogeochemical Cycles
What are Biogeochemical Cycles? ⦿The prefix Bio means living things ⦿Geo means from the Earth or rocks ⦿Chemical is a substance made of elements ⦿A cycle is something that goes around ⦿So a Biogeochemical Cycle is when elements from the Earth are used by living things while they are alive and then returned to the Earth when they die.
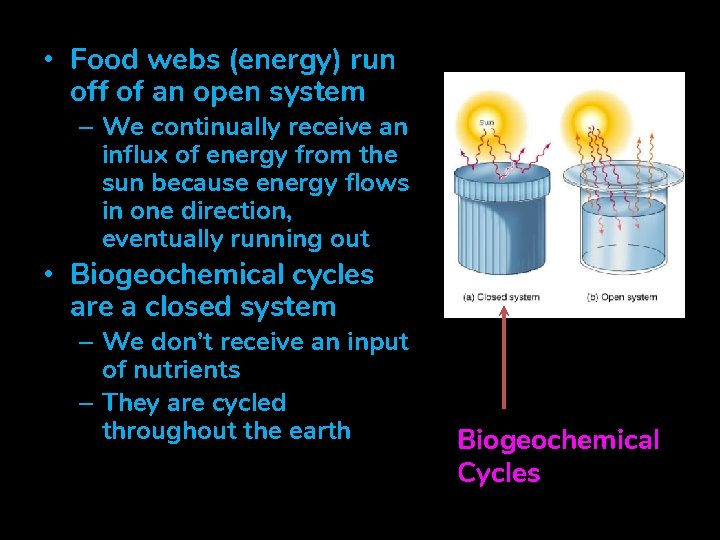
• Food webs (energy) run off of an open system – We continually receive an influx of energy from the sun because energy flows in one direction, eventually running out • Biogeochemical cycles are a closed system – We don’t receive an input of nutrients – They are cycled throughout the earth Biogeochemical Cycles
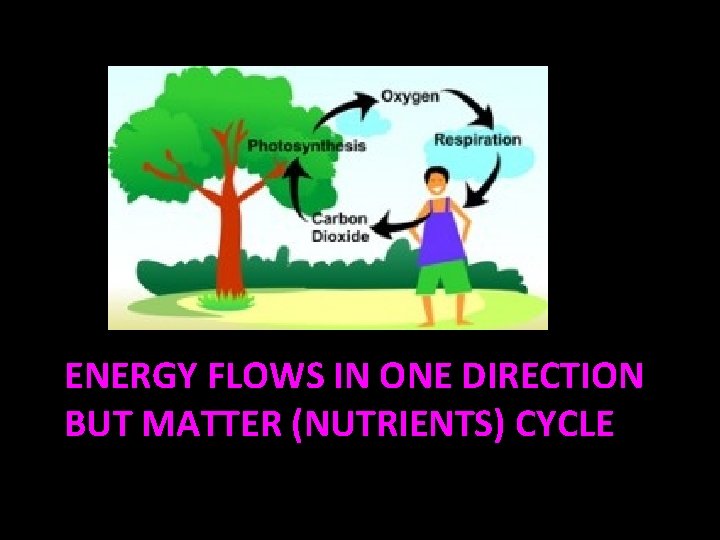
ENERGY FLOWS IN ONE DIRECTION BUT MATTER (NUTRIENTS) CYCLE
WATER CYCLE

Water’s Unique Properties • There are strong forces of attraction between molecules of water (cohesion). • Water exists as a liquid over a wide temperature range. • Liquid water changes temperature slowly. • It takes a large amount of energy for water to evaporate. • Liquid water can dissolve a variety of compounds. • Water expands when it freezes.
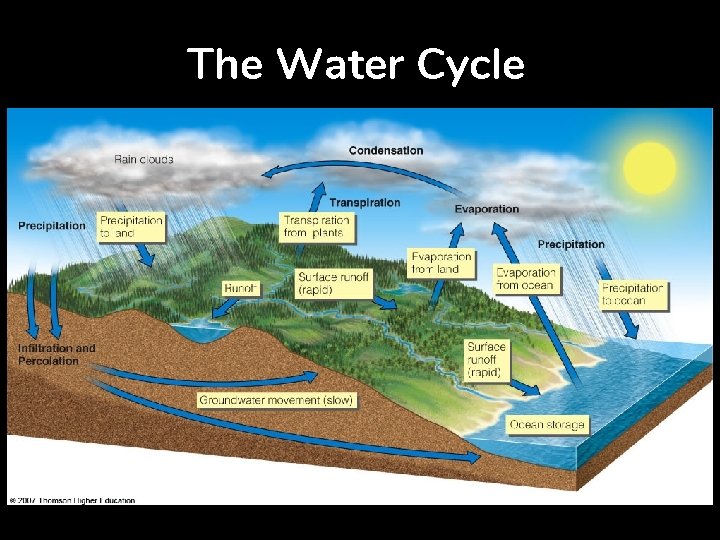
The Water Cycle Figure 3 -26
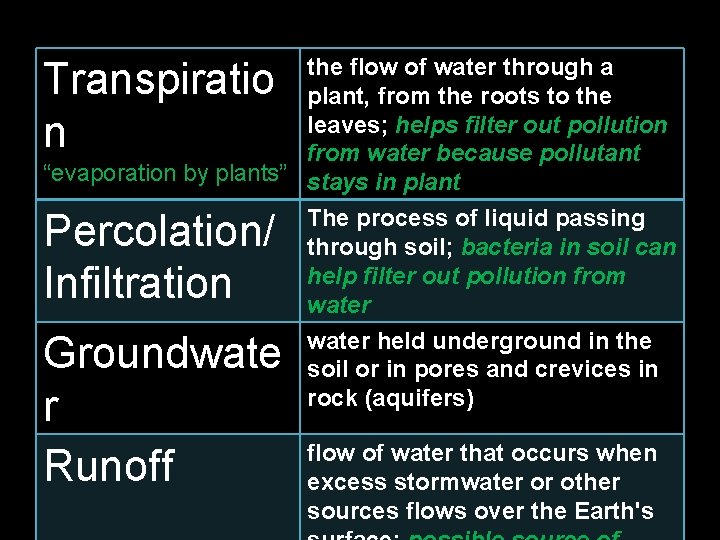
the flow of water through a plant, from the roots to the leaves; helps filter out pollution from water because pollutant “evaporation by plants” stays in plant Transpiratio n Percolation/ Infiltration The process of liquid passing through soil; bacteria in soil can help filter out pollution from water Groundwate r Runoff water held underground in the soil or in pores and crevices in rock (aquifers) flow of water that occurs when excess stormwater or other sources flows over the Earth's

Human Intervention in the hydrologic cycle… • Ground and water depletion • Water pollution – Atmosphere: acid rain – Surface & groundwater pollution • Clearing of vegetation – Interferes with the cycle by decreasing transpiration – Temperate and Tropical Rainforests

CARBON CYCLE
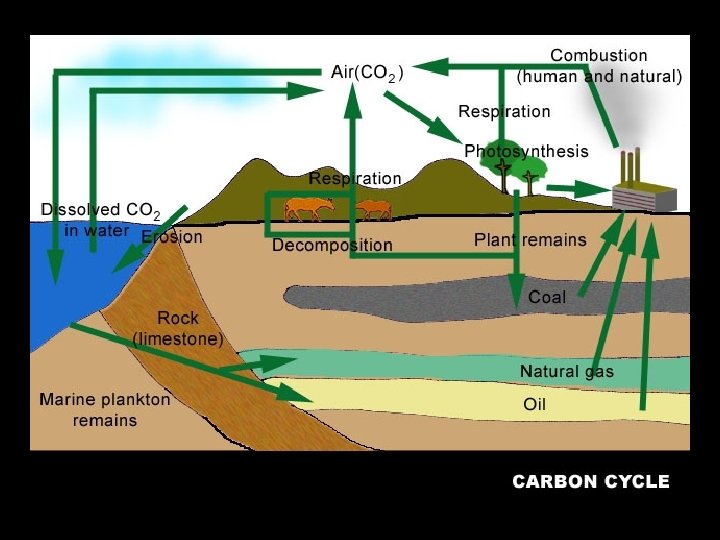
Fig. 3 -27, pp. 72 -
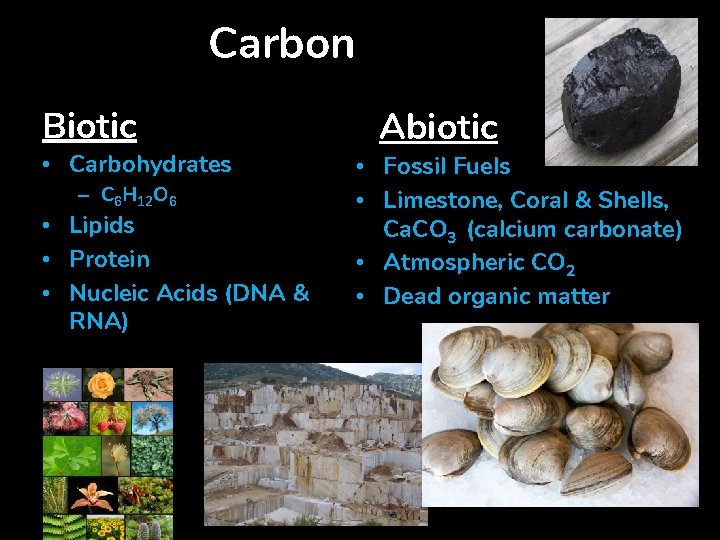
Carbon Biotic • Carbohydrates – C 6 H 12 O 6 • Lipids • Protein • Nucleic Acids (DNA & RNA) Abiotic • Fossil Fuels • Limestone, Coral & Shells, Ca. CO 3 (calcium carbonate) • Atmospheric CO 2 • Dead organic matter
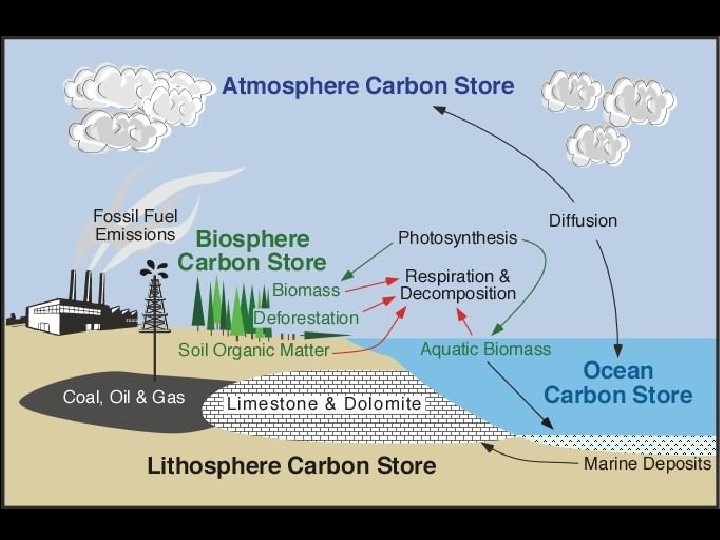

Carbon reservoirs (“sinks”) The atmosphere Carbon dioxide gas The biosphere living things and non-living organic material (*Forests) The oceans dissolved carbon dioxide and the shells of marine animals (hydrosphere) LARGEST SINK/RESERVOIR OF CARBON The lithosphere sedimentary rock formed from shells & skeletons, fossil fuels
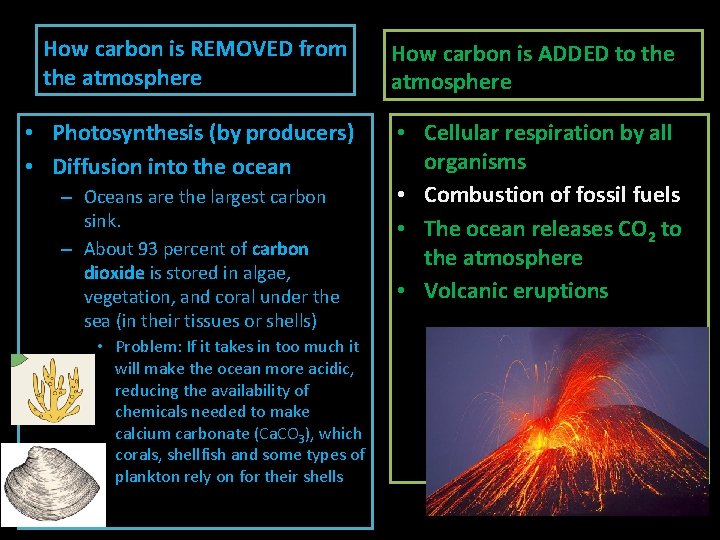
How carbon is REMOVED from the atmosphere How carbon is ADDED to the atmosphere • Photosynthesis (by producers) • Diffusion into the ocean • Cellular respiration by all organisms • Combustion of fossil fuels • The ocean releases CO 2 to the atmosphere • Volcanic eruptions – Oceans are the largest carbon sink. – About 93 percent of carbon dioxide is stored in algae, vegetation, and coral under the sea (in their tissues or shells) • Problem: If it takes in too much it will make the ocean more acidic, reducing the availability of chemicals needed to make calcium carbonate (Ca. CO 3), which corals, shellfish and some types of plankton rely on for their shells
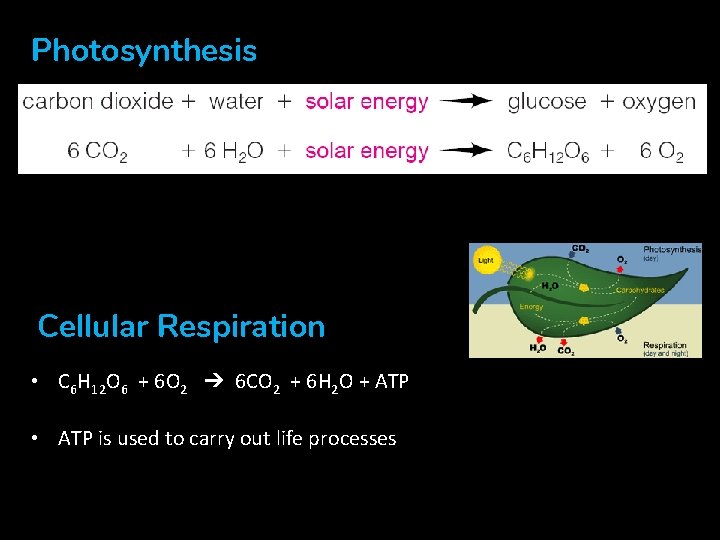
Photosynthesis CO 2 Cellular Respiration • C 6 H 12 O 6 + 6 O 2 ➔ 6 CO 2 + 6 H 2 O + ATP • ATP is used to carry out life processes • + energy

CO 2 enters atmosphere: combustion or oxidization of hydrocarbon (fossil fuels) CH 4 + 2 O 2 ➔ CO 2 + 2 H 2 O + energy 86% of global primary energy consumption is fossil fuels.

Human Intervention in the carbon cycle… • Since the industrial revolution we have dramatically increased CO 2 in our atmosphere due to: – Deforestation • Less plants available to remove CO 2 for photosynthesis – Forest fires • Burning trees return carbon in the biomass of the forest to the atmosphere – Increased combustion of fossil fuels

PHOSPHORUS CYCLE
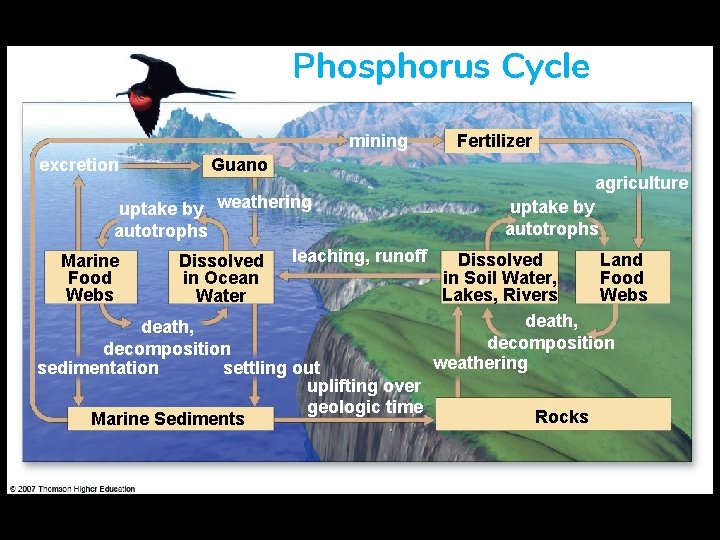
Phosphorus Cycle mining excretion Guano Fertilizer agriculture uptake by weathering autotrophs leaching, runoff Dissolved Land Marine Dissolved in Soil Water, Food in Ocean Lakes, Rivers Webs Water death, decomposition weathering sedimentation settling out uplifting over geologic time Rocks Marine Sediments Fig. 3 -31, p. 77
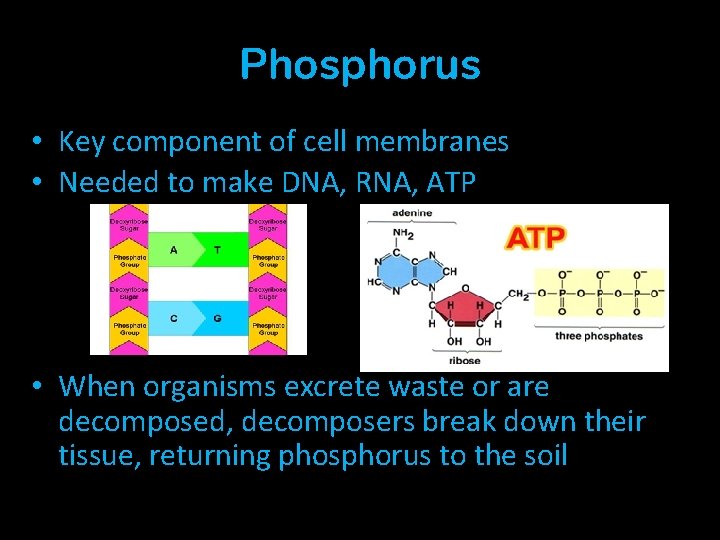
Phosphorus • Key component of cell membranes • Needed to make DNA, RNA, ATP • When organisms excrete waste or are decomposed, decomposers break down their tissue, returning phosphorus to the soil

Phosphorus: a slow cycle • 1. No atmospheric component • 2. Vast majority of phosphorus if contained within rock & can only be released by weathering, which releases PO 43 - (phosphate) into water. – Plants take up phosphate dissolved in water through their roots – Phosphates dissolved in lakes/oceans precipitate into solid form & settle to the bottom • Insoluble form that is unavailable until its weathered • Because of these reasons, phosphorus availability to organisms tends to be low, making it a limiting factor

Human Influence on Phosphorus Cycle • Mining – Becomes runoff pollution • Deforestation – Removing vegetation lowers P availability in the ecosystem (no longer decomposed) • Eutrophication – Source: Agricultural runoff (feces & fertilizer) & sewage • Phosphates are present in detergents – Purchase phosphate-free

SULFUR CYCLE
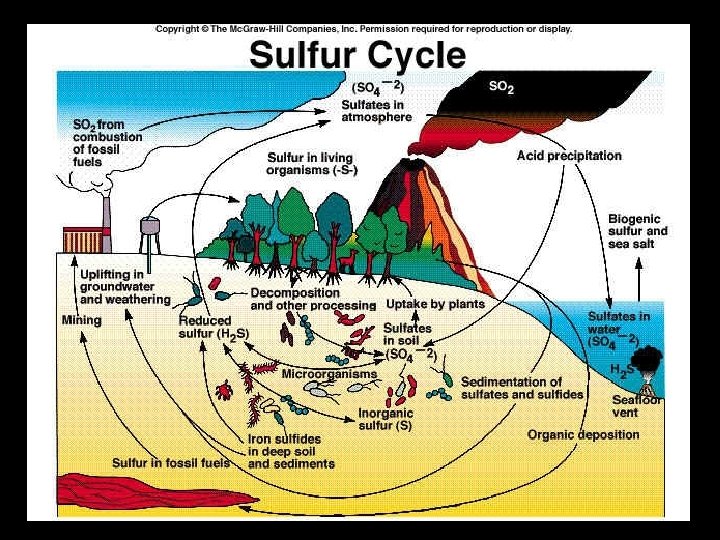

Sulfur Cycle • Used by living things to construct proteins • Most sulfur is found in rocks & is released by weathering & volcanic activity – Iron sulfide (pyrite) – Calcium sulfate (gypsum) • Sulfate ions are taken up by producers, passed to consumers, and released again after decomposition
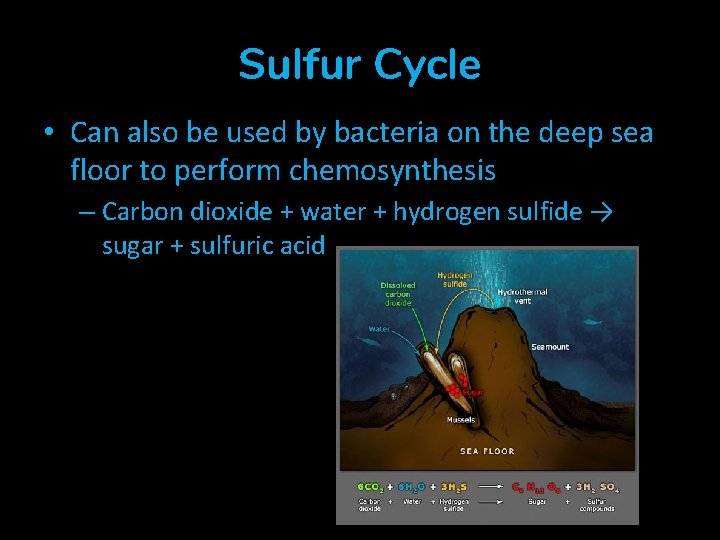
Sulfur Cycle • Can also be used by bacteria on the deep sea floor to perform chemosynthesis – Carbon dioxide + water + hydrogen sulfide → sugar + sulfuric acid

Human Influence on Sulfur Cycle • Burning coal produces sulfur dioxide, SO 2 – SO 2 forms sulfuric acid, H 2 SO 4, in the atmosphere and results in acidification of ecosystems and smog formation • Coal mining – Releases sulfur→ runoff may damage aquatic ecosystems • Refining petroleum & smelting (extracting metal) – Releases sulfur compounds into the atmosphere • Volcanic eruptions release sulfate aerosols SO 42 -, which cause global cooling
NITROGEN CYCLE
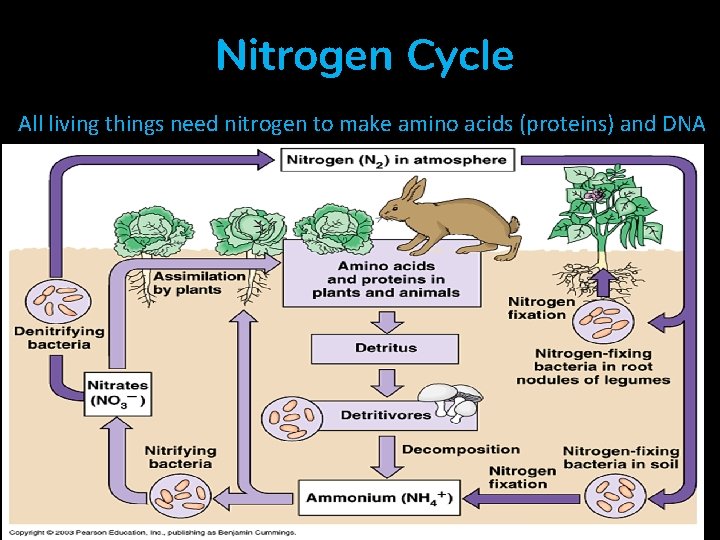
Nitrogen Cycle All living things need nitrogen to make amino acids (proteins) and DNA

Nitrogen Cycle • Makes up 78% of the atmosphere, N 2 • Plants can’t use this form of nitrogen (N 2) • It needs to be converted into ammonium, NH 4+ or nitrate, NO 3– Nitrogen fixation • Lightening, bacteria (in legume roots), humans (application of fertilizer) – Nitrification • Bacteria
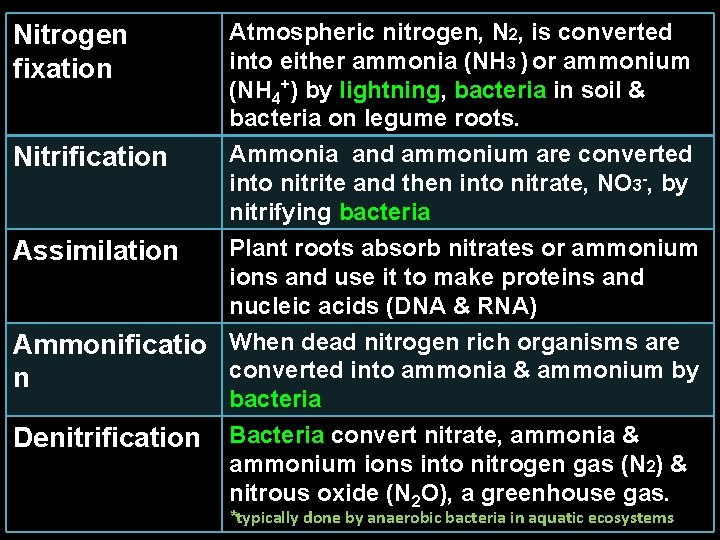
Nitrogen fixation Atmospheric nitrogen, N 2, is converted into either ammonia (NH 3 ) or ammonium (NH 4+) by lightning, bacteria in soil & bacteria on legume roots. Nitrification Ammonia and ammonium are converted into nitrite and then into nitrate, NO 3 -, by nitrifying bacteria Assimilation Plant roots absorb nitrates or ammonium ions and use it to make proteins and nucleic acids (DNA & RNA) Ammonificatio When dead nitrogen rich organisms are converted into ammonia & ammonium by n bacteria Denitrification Bacteria convert nitrate, ammonia & ammonium ions into nitrogen gas (N 2) & nitrous oxide (N 2 O), a greenhouse gas. *typically done by anaerobic bacteria in aquatic ecosystems

Forms of nitrogen easiest to uptake by Plants 1. NITRATE 2. AMMONIUM
Legumes & N-fixing bacteria: mutualistic relationship • Nitrogen-fixing bacteria live in the root nodules of legume plants • Allows farmers to reduce fertilizer use because the symbiotic relationship increases nitrogen fixation Soybeans, peas, clover, alfalfa
Haber-Bosch method • German scientists figure out how to fix nitrogen on an industrial scale • Nitrogen and hydrogen gas are synthesized to make ammonia • We have doubled the natural rate of nitrogen fixation

Human Influence on Nitrogen Cycle • Fossil Fuel Combustion • NO is released→ forms nitric acid in atmosphere and results in acidification of ecosystems and smog formation • N 2 O gas is a greenhouse gas • Source: livestock waste & use of fertilizer • Deforestation • Nitrogen is removed from an ecosystem (no longer decomposed) • Eutrophication – Source: Agricultural runoff (animal waste & fertilizer) & sewage (human waste)
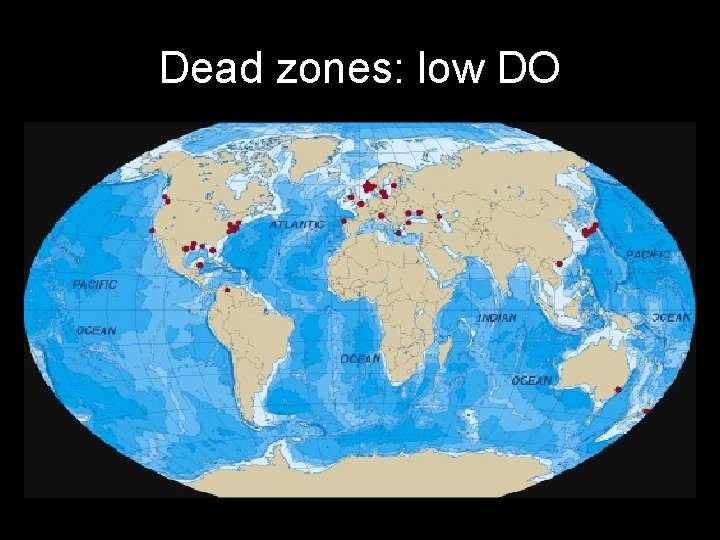
Dead zones: low DO

• What causes these Dead Zones/Hypoxia? EUTROPHICATION

Nutrient Pollution • Hypoxia: extremely low dissolved oxygen (DO) concentrations in a body of water • The effect: “Dead Zones”: dramatic changes in the aquatic ecosystem as organisms in water will not have enough oxygen to survive

Eutrophication • 1. Excess phosphates or nitrates enter surface water – Runoff from farms (fertilizer & animal waste) – Golf courses, lawns (fertilizer) – Runoff from sewage (human waste) • 2. Nutrients fertilizes algae & aquatic plants, increasing their growth rates & populations (algal bloom) • 3. They provide O 2 & food for other organisms, but at the same time they can cover the water’s surface, blocking out sunlight to deeper-water plants • 4. As algae die, they provide food for decomposing bacteria • 5. Decomposition requires oxygen, so the increase in bacterial activity decreases levels of dissolved oxygen • 6. These levels can drop too low to support fish & shellfish

Oligotrophic • Low-nutrient • High-oxygen Eutrophic • High-nutrient • Low-oxygen • Caused by eutrophication
- Slides: 42