Negative Thermal Expansion By The Team Negative thermal
Negative Thermal Expansion By ‘The Team’
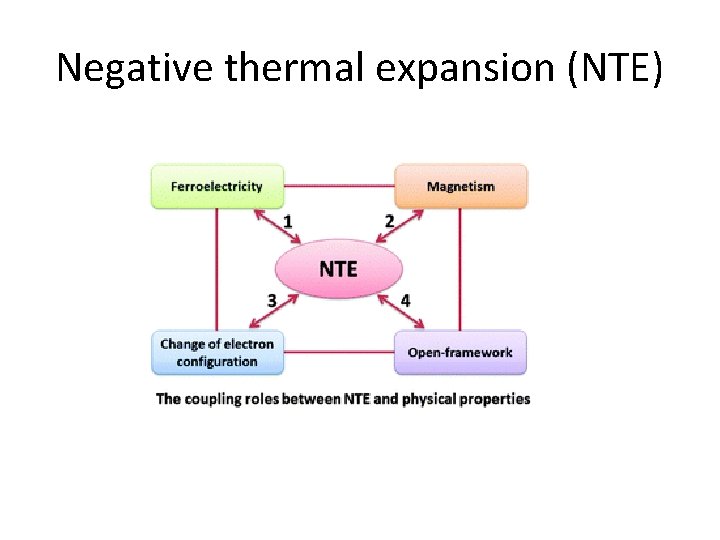
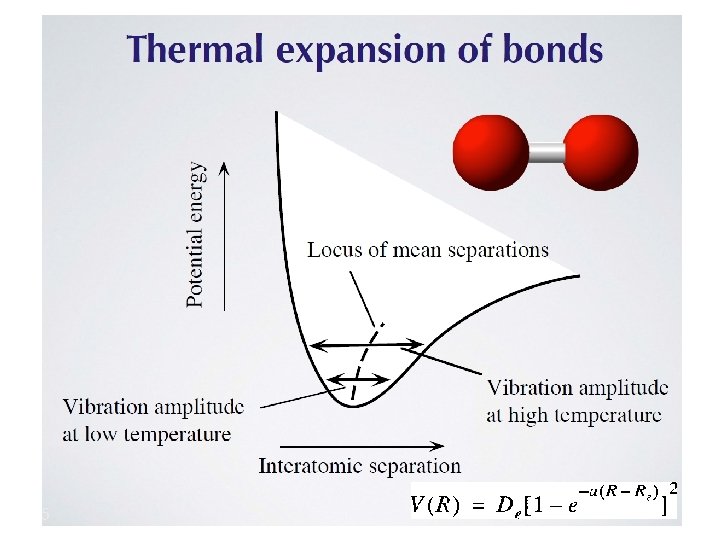
Negative thermal expansion (NTE)
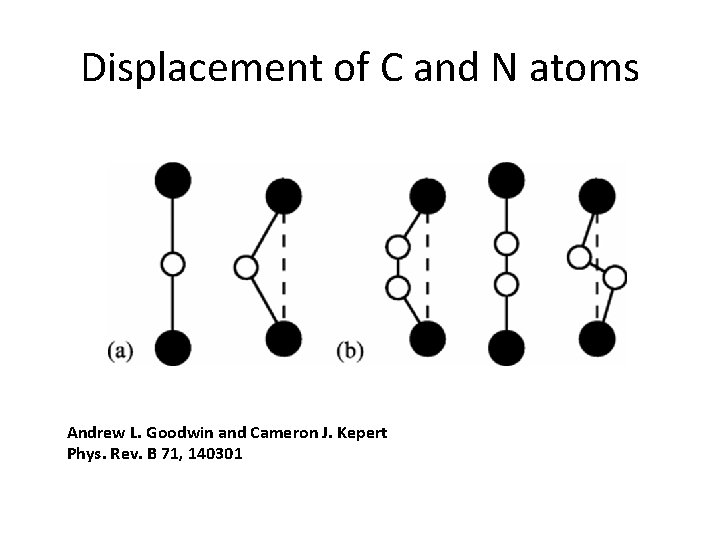
Displacement of C and N atoms Andrew L. Goodwin and Cameron J. Kepert Phys. Rev. B 71, 140301
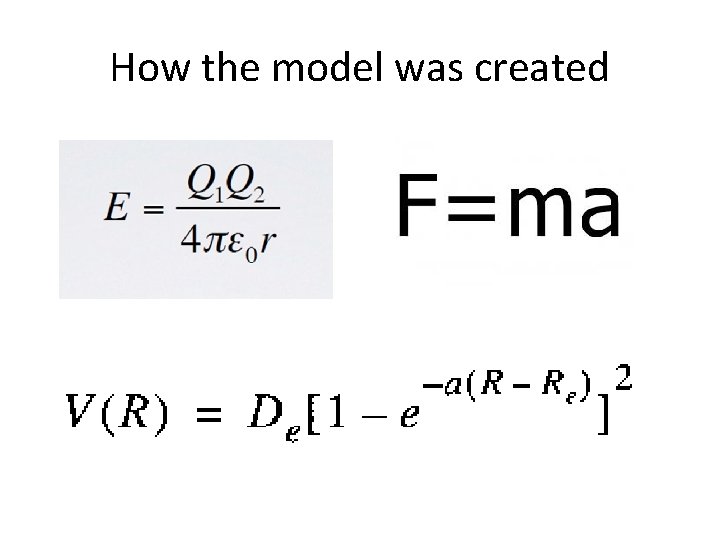
How the model was created
Computer modelling of the lattice
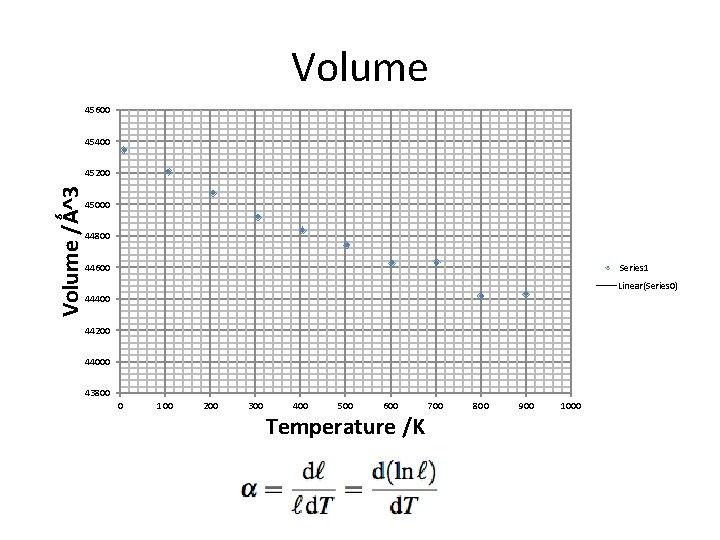
Volume 45600 45400 Volume /Ǻ^3 45200 45000 44800 Series 1 44600 Linear(Series 0) 44400 44200 44000 43800 0 100 200 300 400 500 600 Temperature /K 700 800 900 1000
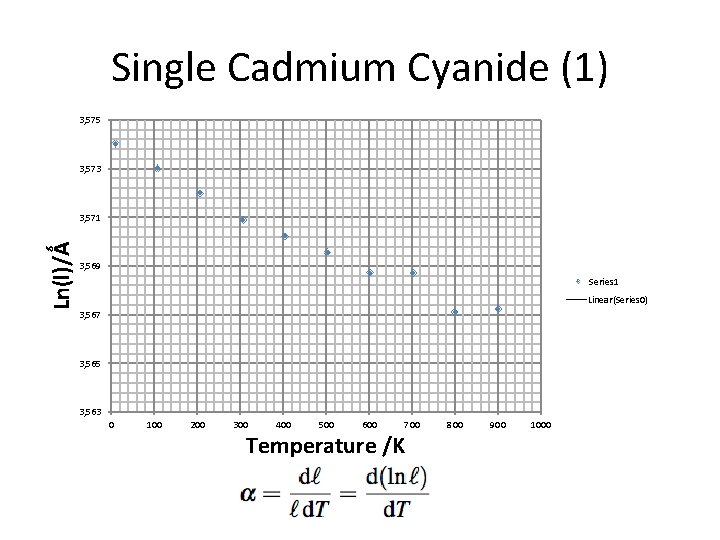
Single Cadmium Cyanide (1) 3, 575 3, 573 Ln(l)/Ǻ 3, 571 3, 569 Series 1 Linear(Series 0) 3, 567 3, 565 3, 563 0 100 200 300 400 500 600 700 Temperature /K 800 900 1000
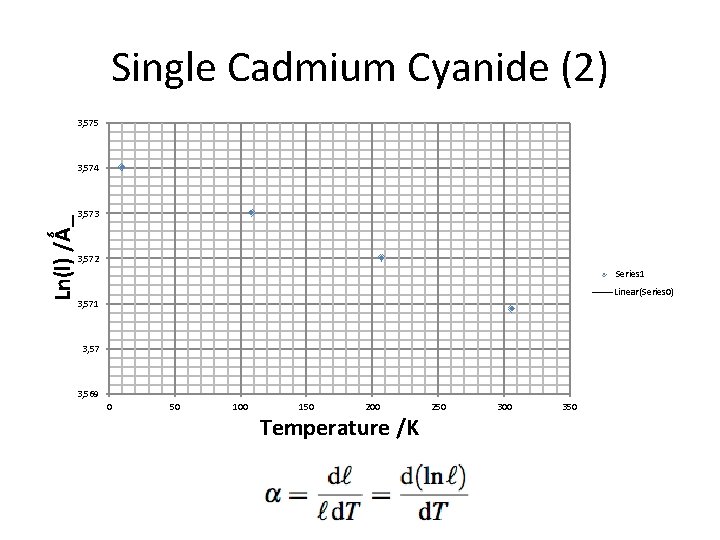
Single Cadmium Cyanide (2) 3, 575 Ln(l) /Ǻ_ 3, 574 3, 573 3, 572 Series 1 Linear(Series 0) 3, 571 3, 57 3, 569 0 50 100 150 200 Temperature /K 250 300 350
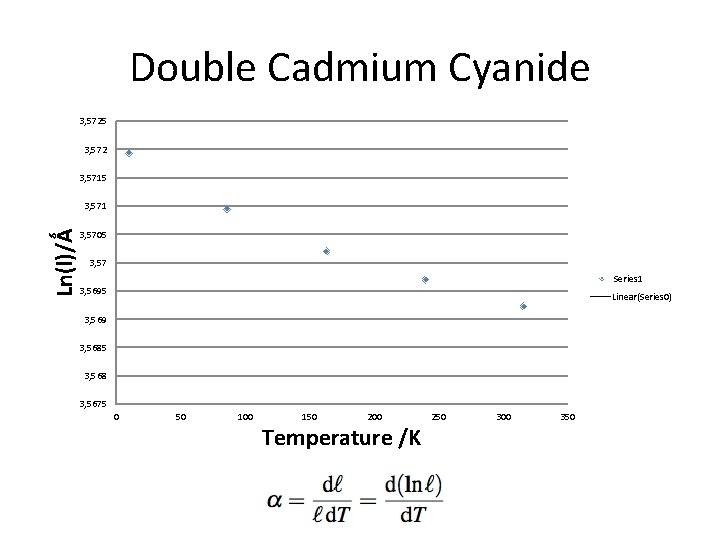
Double Cadmium Cyanide 3, 5725 3, 572 3, 5715 Ln(l)/Ǻ 3, 571 3, 5705 3, 57 Series 1 3, 5695 Linear(Series 0) 3, 569 3, 5685 3, 568 3, 5675 0 50 100 150 200 Temperature /K 250 300 350
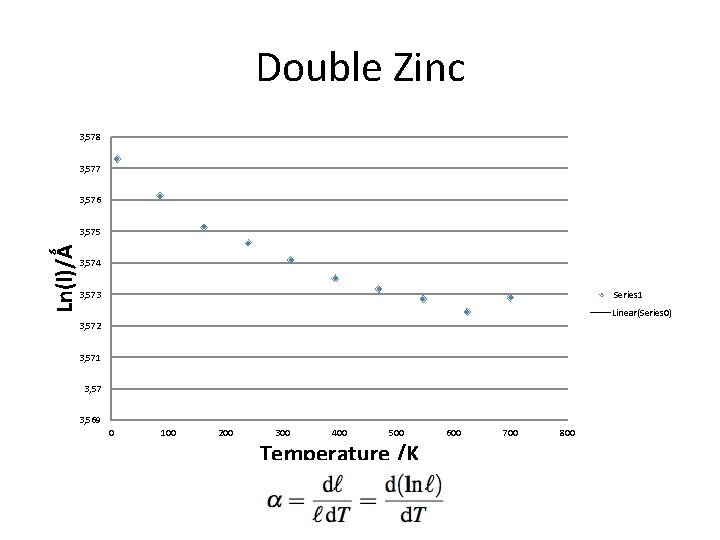
Double Zinc 3, 578 3, 577 3, 576 Ln(l)/Ǻ 3, 575 3, 574 Series 1 3, 573 Linear(Series 0) 3, 572 3, 571 3, 57 3, 569 0 100 200 300 400 500 Temperature /K 600 700 800
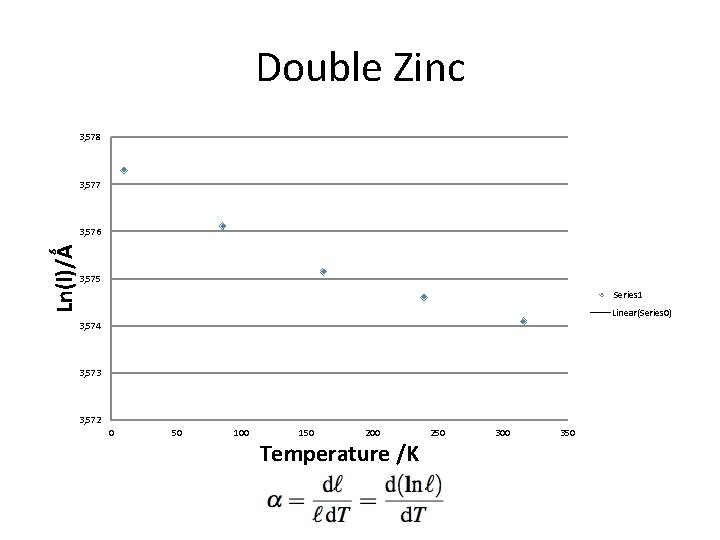
Double Zinc 3, 578 3, 577 Ln(l)/Ǻ 3, 576 3, 575 Series 1 Linear(Series 0) 3, 574 3, 573 3, 572 0 50 100 150 200 Temperature /K 250 300 350
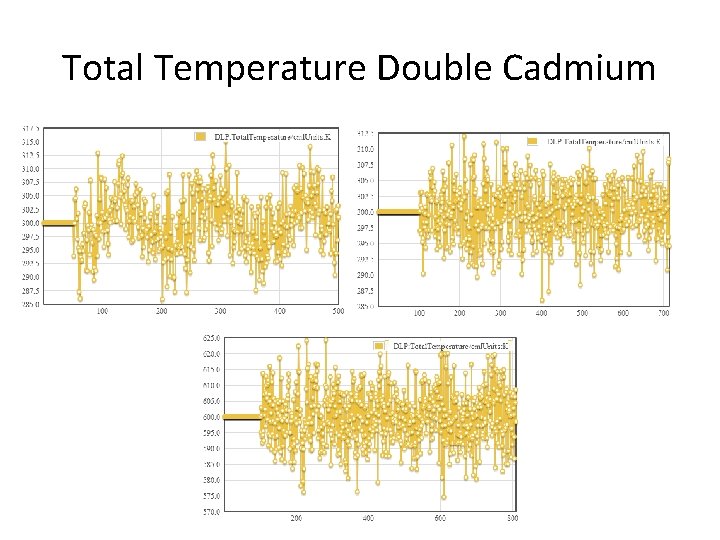
Total Temperature Double Cadmium
Effects of Pressure • As temperature increased , we would expect a decrease in volume (NTE) • The extent of NTE also varies with pressure • We observed the impact of systematic increases of pressure on volume • We expect further NTE with increased pressure over the set volume range
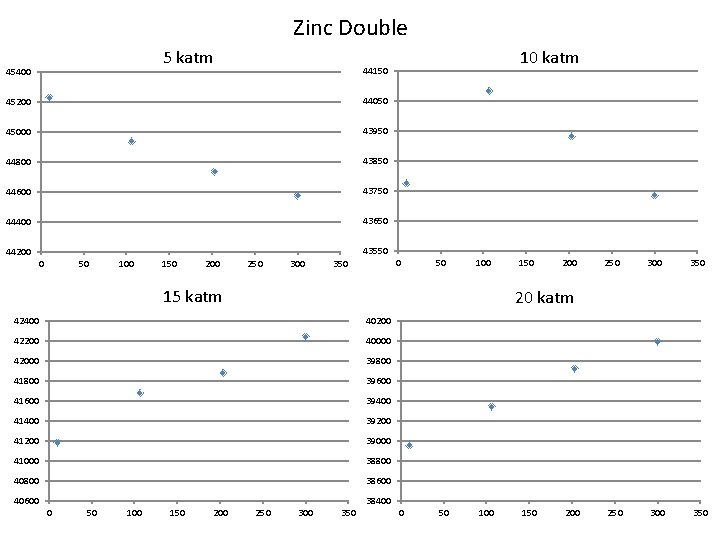
Zinc Double 5 katm 45400 10 katm 44150 45200 44050 45000 43950 44800 43850 44600 43750 44400 43650 43550 44200 0 50 100 150 200 250 300 0 350 50 100 15 katm 40200 42200 40000 42000 39800 41800 39600 41600 39400 41400 39200 41200 39000 41000 38800 40800 38600 40600 38400 50 100 150 200 250 300 350 20 katm 42400 0 150 250 300 350 0 50 100 150 200
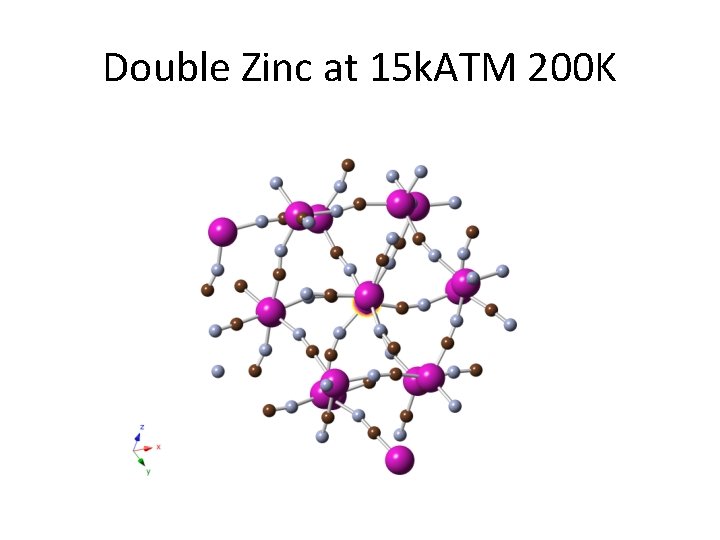
Double Zinc at 15 k. ATM 200 K
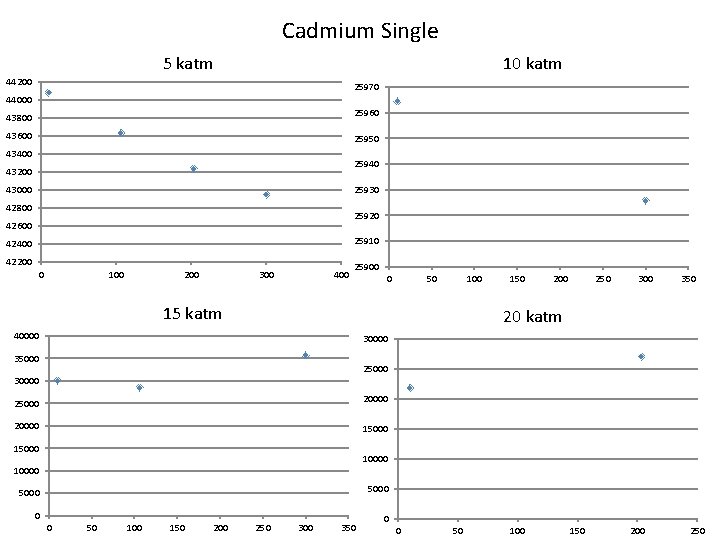
Cadmium Single 10 katm 5 katm 44200 25970 44000 25960 43800 43600 25950 43400 25940 43200 25930 43000 42800 25920 42600 25910 42400 42200 0 100 200 300 400 25900 0 50 100 15 katm 150 200 250 300 350 20 katm 40000 35000 25000 30000 25000 20000 15000 10000 5000 0 0 50 100 150 200 250 300 350 0 0 50 100 150 200 250
Conclusions • The simulation for both Cadmium and Zinc single configurations are unstable above 5 katm, resulting in implosion • The double structures responded as an NTE, until a critical point whereby they began behaving as normal materials (increasing in volume)
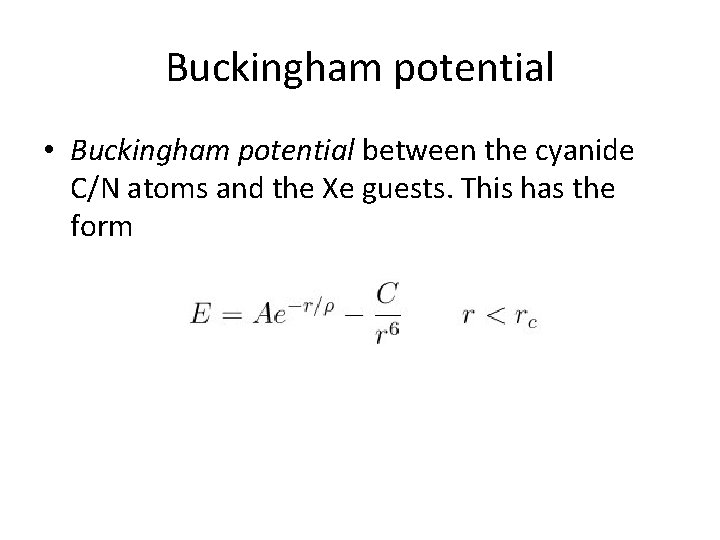
Buckingham potential • Buckingham potential between the cyanide C/N atoms and the Xe guests. This has the form
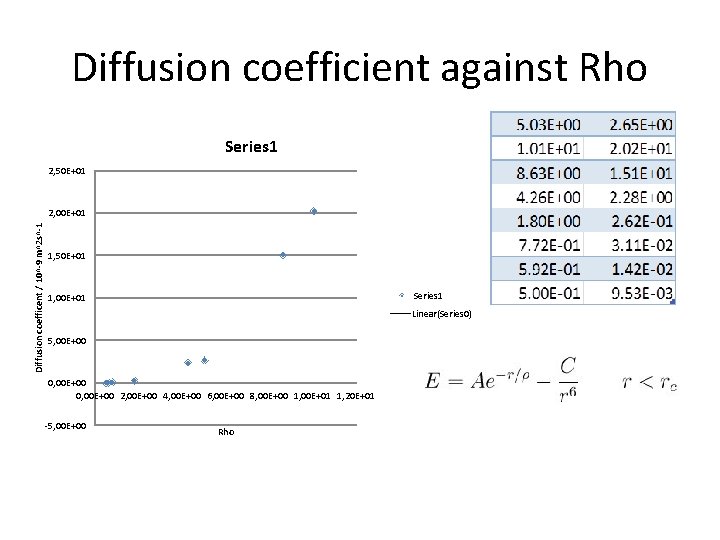
Diffusion coefficient against Rho Series 1 2, 50 E+01 Diffusion coefficent / 10^-9 m^2 s^-1 2, 00 E+01 1, 50 E+01 Series 1 1, 00 E+01 Linear(Series 0) 5, 00 E+00 0, 00 E+00 2, 00 E+00 4, 00 E+00 6, 00 E+00 8, 00 E+00 1, 00 E+01 1, 20 E+01 -5, 00 E+00 Rho
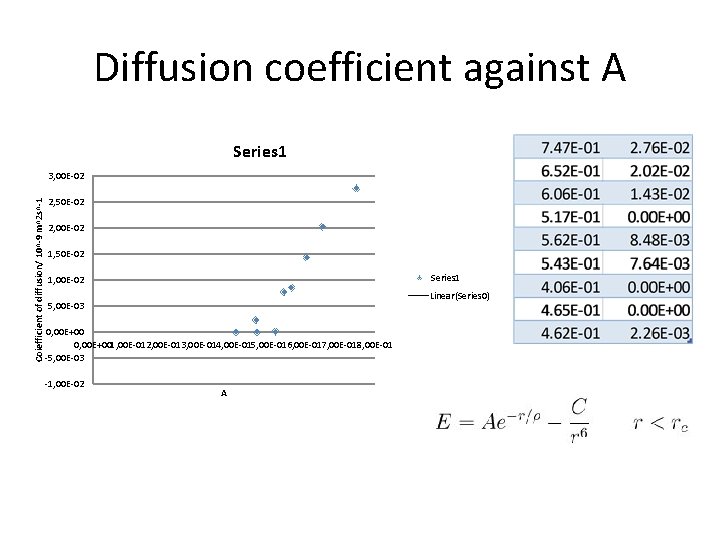
Diffusion coefficient against A Series 1 Coiefficient of diffusion/ 10^-9 m^2 s^-1 3, 00 E-02 2, 50 E-02 2, 00 E-02 1, 50 E-02 Series 1 1, 00 E-02 Linear(Series 0) 5, 00 E-03 0, 00 E+001, 00 E-012, 00 E-013, 00 E-014, 00 E-015, 00 E-016, 00 E-017, 00 E-018, 00 E-01 -5, 00 E-03 -1, 00 E-02 A

Uses
Further development

- Slides: 24