Nearinfrared spectroscopy of ethynyl radical C 2 H



- Slides: 21

Near-infrared spectroscopy of ethynyl radical, C 2 H ANH T. LE, GREGORY HALL, TREVOR SEARSa Division of Chemistry Department of Energy and Photon Sciences Brookhaven National Laboratory Upton, NY, USA International Symposium on Molecular Spectroscopy 71 st Meeting, June 19 -24, 2016 RF 05 Champaign-Urbana, Illinois a. Also Department of Chemistry, Stony Brook University, Stony Brook, New York 11794

Motivation Ethynyl plays an important role in both processes forming Carbon nanotubes and soot formation Also a main photodissociation product of the acetylene which is ejected (via strong stellar winds) into the outer envelope of carbon-rich evolved stars. C 2 H in interstellar medium • • • Tucker et. al. Astrophys. J. 193 L 115–L 119 Wootten et. al. Astrophys. J. 239 944– 854 (1980). Nyman, Astron. Astrophys. J. 141 323– 327 (1984). Combes et. al. Astrophys. J. 147 L 25–L 26 (1985) Saleck et. al. Can. J. Phys. 72 747– 754 (1994) Keady and K. H. Hinkle, Astrophys. J. 331 539– 546 (1988) Tenenbaum et. al. Astrophys. J. 720 L 102–L 107 (2010) (1974)

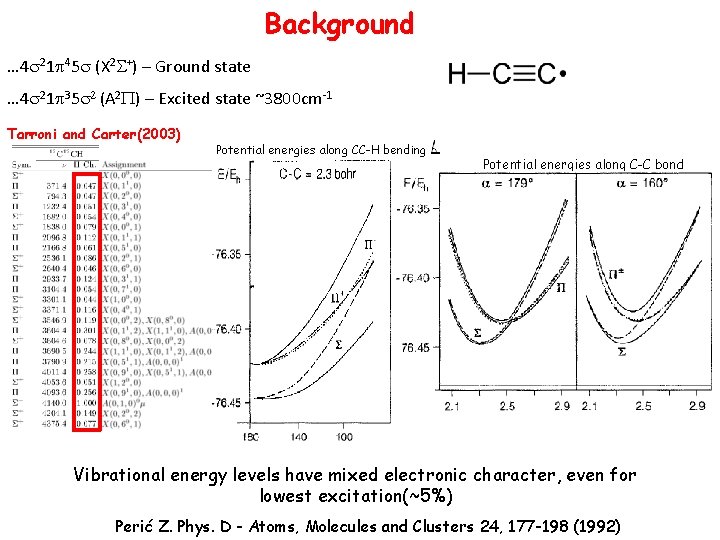
Background … 4 s 21 p 45 s (X 2 S+) – Ground state … 4 s 21 p 35 s 2 (A 2 P) – Excited state ~3800 cm-1 Tarroni and Carter(2003) Potential energies along CC-H bending Potential energies along C-C bond Vibrational energy levels have mixed electronic character, even for lowest excitation(~5%) Perić Z. Phys. D - Atoms, Molecules and Clusters 24, 177 -198 (1992)

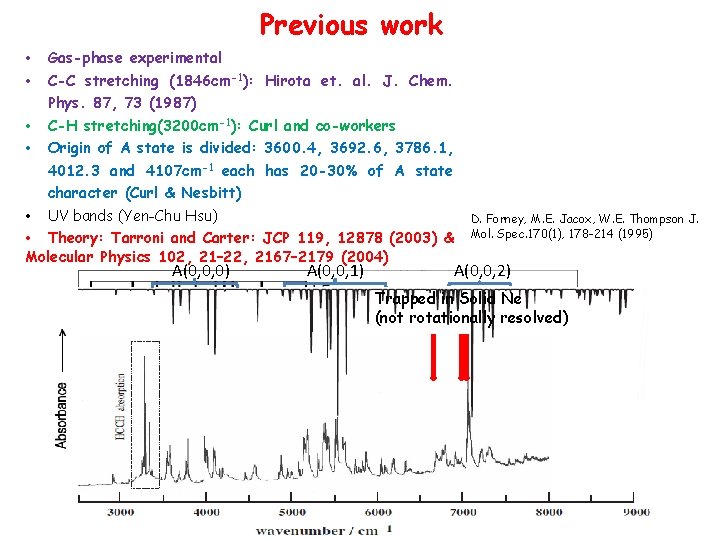
Previous work • • Gas-phase experimental C-C stretching (1846 cm-1): Hirota et. al. J. Chem. Phys. 87, 73 (1987) • C-H stretching(3200 cm-1): Curl and co-workers • Origin of A state is divided: 3600. 4, 3692. 6, 3786. 1, 4012. 3 and 4107 cm-1 each has 20 -30% of A state character (Curl & Nesbitt) • UV bands (Yen-Chu Hsu) • Theory: Tarroni and Carter: JCP 119, 12878 (2003) & Molecular Physics 102, 21– 22, 2167– 2179 (2004) A(0, 0, 0) A(0, 0, 1) D. Forney, M. E. Jacox, W. E. Thompson J. Mol. Spec. 170(1), 178 -214 (1995) A(0, 0, 2) Trapped in Solid Ne (not rotationally resolved)

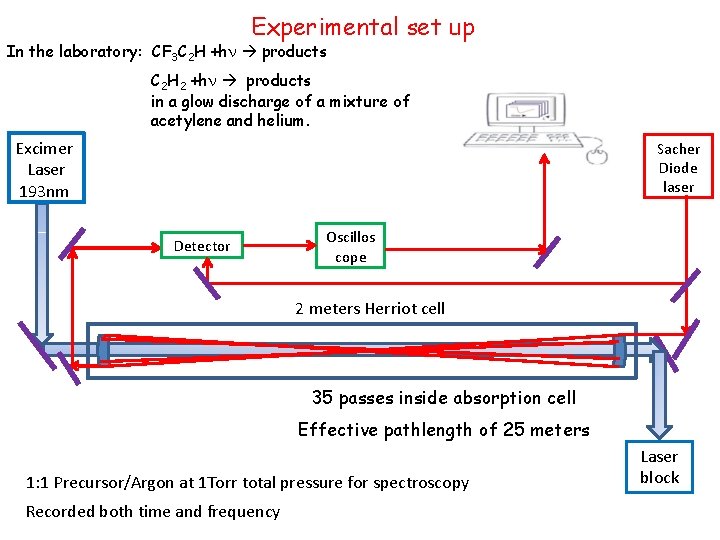
Experimental set up In the laboratory: CF 3 C 2 H +hn products C 2 H 2 +hn products in a glow discharge of a mixture of acetylene and helium. Excimer Laser 193 nm Sacher Diode laser Detector Oscillos cope 2 meters Herriot cell 35 passes inside absorption cell Effective pathlength of 25 meters 1: 1 Precursor/Argon at 1 Torr total pressure for spectroscopy Recorded both time and frequency Laser block

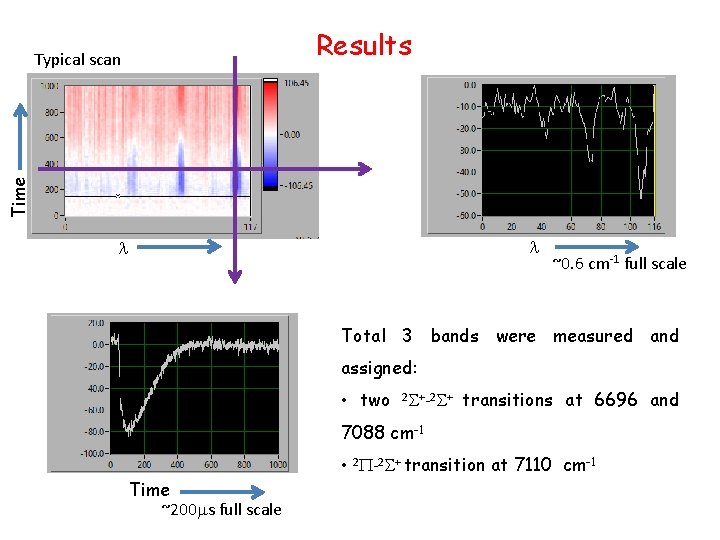
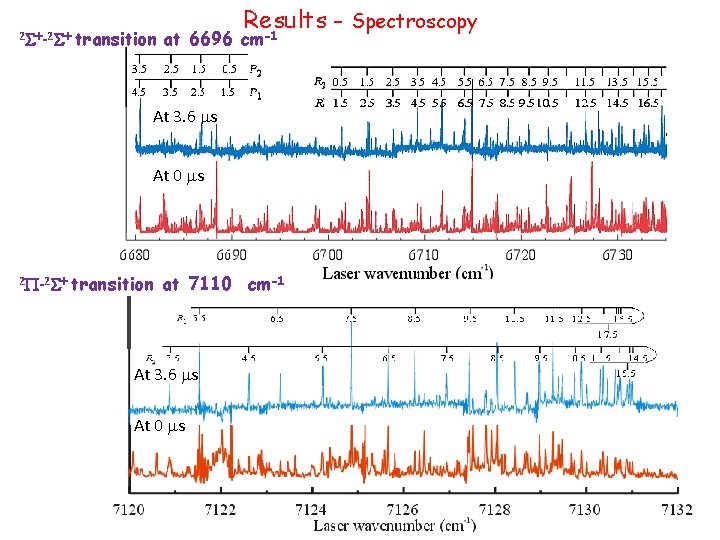
Results Time Typical scan l l ~0. 6 cm-1 full scale Total 3 bands were measured and assigned: • two 2 S+-2 S+ transitions at 6696 and 7088 cm-1 • 2 P-2 S+ transition at 7110 cm-1 Time ~200 ms full scale

2 S+-2 S+ Results - Spectroscopy -1 transition at 6696 cm At 3. 6 ms At 0 ms 2 P-2 S+ transition at 7110 cm-1 At 3. 6 ms At 0 ms

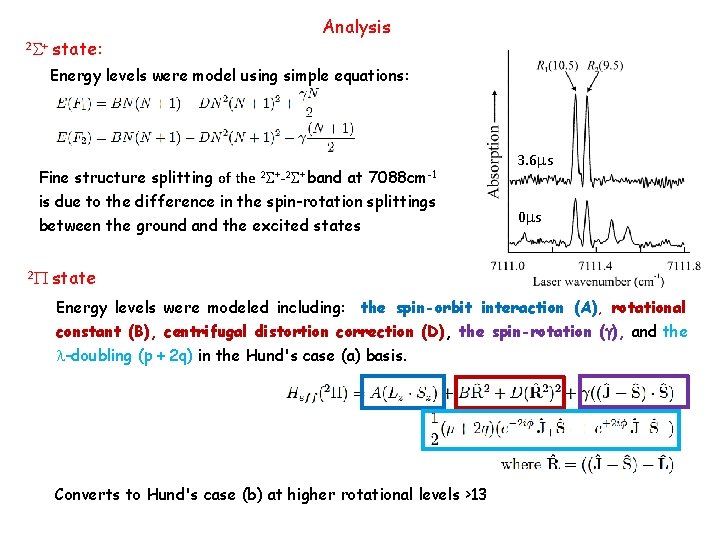
2 S+ state: Analysis Energy levels were model using simple equations: Fine structure splitting of the 2 S+-2 S+ band at 7088 cm-1 is due to the difference in the spin-rotation splittings between the ground and the excited states 2 P 3. 6 ms 0 ms state Energy levels were modeled including: the spin-orbit interaction (A), rotational constant (B), centrifugal distortion correction (D), the spin-rotation (g), and the l-doubling (p + 2 q) in the Hund's case (a) basis. Converts to Hund's case (b) at higher rotational levels >13

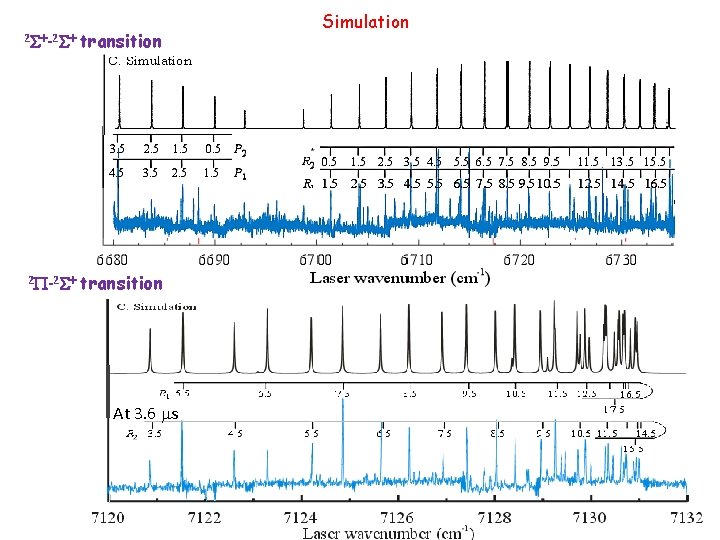
2 S+-2 S+ transition 2 P-2 S+ transition At 3. 6 ms Simulation

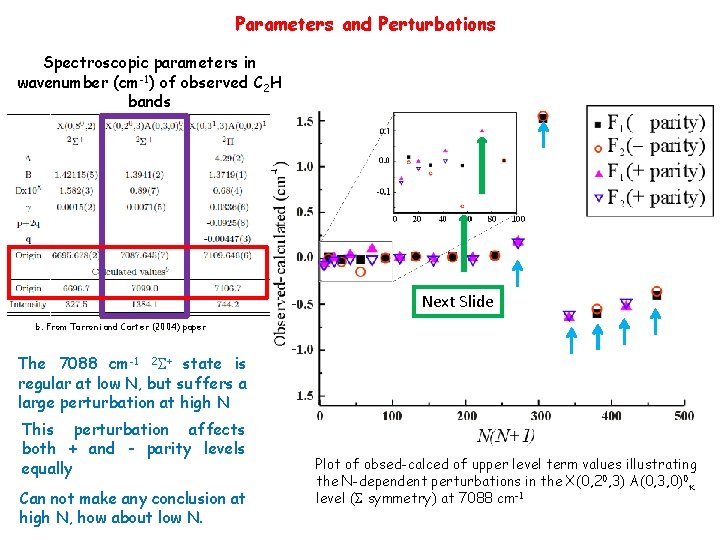
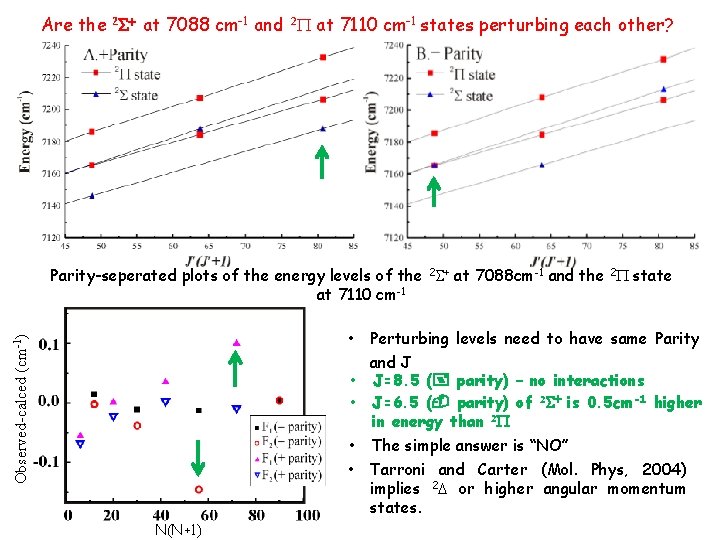
Parameters and Perturbations Spectroscopic parameters in wavenumber (cm-1) of observed C 2 H bands Next Slide b. From Tarroni and Carter (2004) paper The 7088 cm-1 2 S+ state is regular at low N, but suffers a large perturbation at high N This perturbation affects both + and - parity levels equally Can not make any conclusion at high N, how about low N. Plot of obsed-calced of upper level term values illustrating the N-dependent perturbations in the X(0, 20, 3) A(0, 3, 0)0 k level (S symmetry) at 7088 cm-1

Are the 2 S+ at 7088 cm-1 and 2 P at 7110 cm-1 states perturbing each other? Parity-seperated plots of the energy levels of the 2 S+ at 7088 cm-1 and the 2 P state at 7110 cm-1 Perturbing levels need to have same Parity and J • J=8. 5 (+ parity) – no interactions • J=6. 5 (- parity) of 2 S+ is 0. 5 cm-1 higher in energy than 2 P • The simple answer is “NO” Observed-calced (cm-1) • • N(N+1) Tarroni and Carter (Mol. Phys, 2004) implies 2 D or higher angular momentum states.

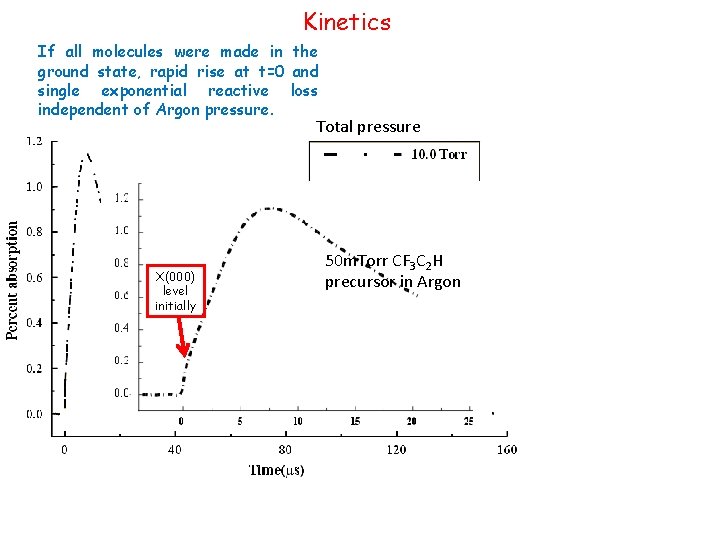
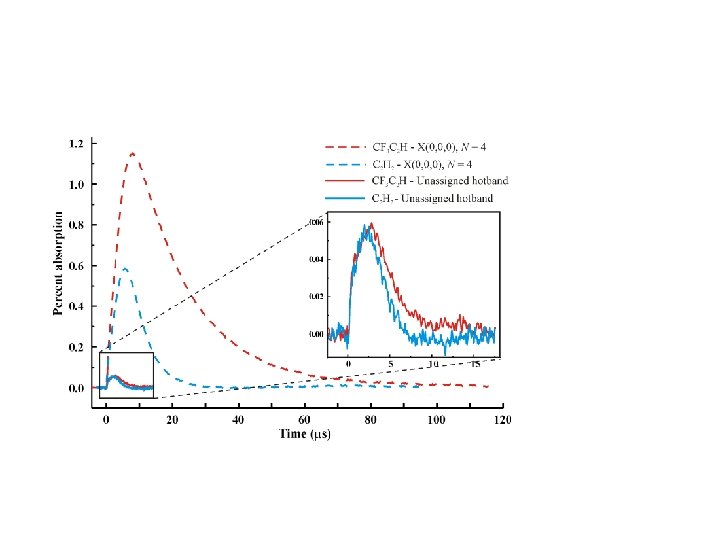
Kinetics If all molecules were made in the ground state, rapid rise at t=0 and single exponential reactive loss independent of Argon pressure. Total pressure X(000) level initially 50 m. Torr CF 3 C 2 H precursor in Argon

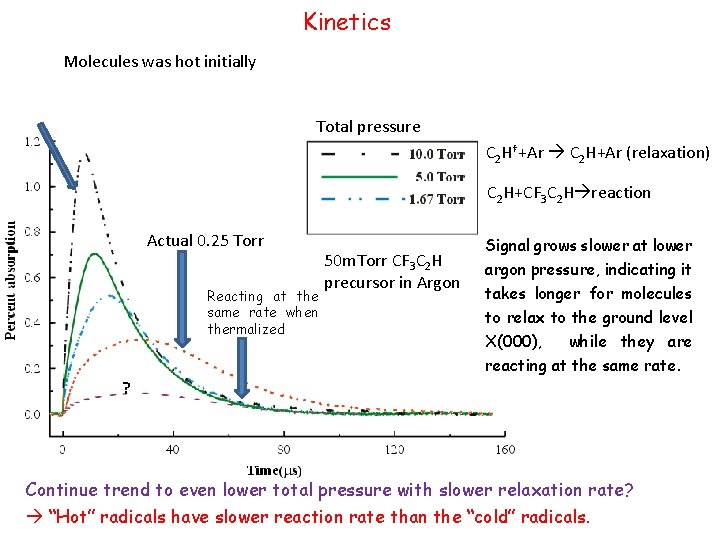
Kinetics Molecules was hot initially Total pressure C 2 H†+Ar C 2 H+Ar (relaxation) C 2 H+CF 3 C 2 H reaction Actual 0. 25 Torr Reacting at the same rate when thermalized 50 m. Torr CF 3 C 2 H precursor in Argon Signal grows slower at lower argon pressure, indicating it takes longer for molecules to relax to the ground level X(000), while they are reacting at the same rate. ? Continue trend to even lower total pressure with slower relaxation rate? “Hot” radicals have slower reaction rate than the “cold” radicals.

Conclusion Spectroscopy • Spectroscopic parameters for 3 bands at 6696, 7088 and 7109 cm-1 are determined. • Origins and intensity ratio between three bands are in good agreement with Tarroni and Carter (Molecular Physics (2004)) • PES complex, X state is highly mixed with A state. • Many unassigned transitions originate from higher vibrational levels of X state. Kinetics • Non-Thermal reaction rate relaxation rate vs reaction rate. • Level dependence of reaction rates

Acknowledgements Anh Le Hong Xu Sylvestre Twagirayezu Trevor Sears Greg Hall Thank you May 2016 Contract No. DE-SC 0012704

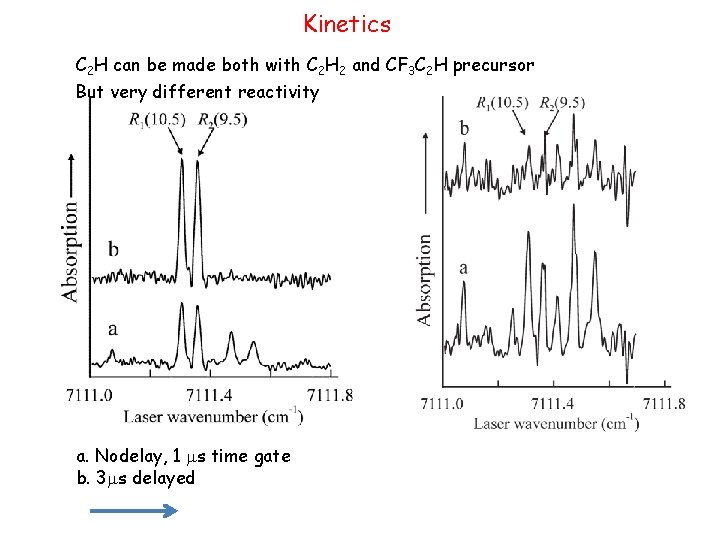
Kinetics C 2 H can be made both with C 2 H 2 and CF 3 C 2 H precursor But very different reactivity a. Nodelay, 1 ms time gate b. 3 ms delayed

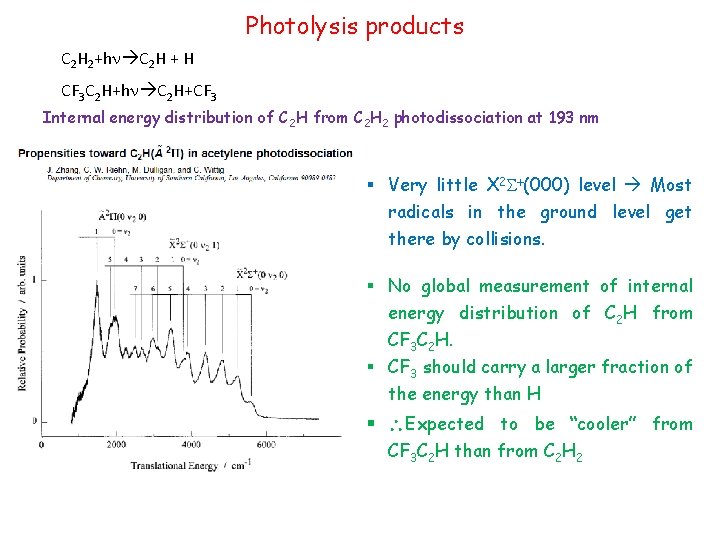
Photolysis products C 2 H 2+hn C 2 H + H CF 3 C 2 H+hn C 2 H+CF 3 Internal energy distribution of C 2 H from C 2 H 2 photodissociation at 193 nm § Very little X 2 S+(000) level Most radicals in the ground level get there by collisions. § No global measurement of internal energy distribution of C 2 H from CF 3 C 2 H. § CF 3 should carry a larger fraction of the energy than H § Expected to be “cooler” from CF 3 C 2 H than from C 2 H 2

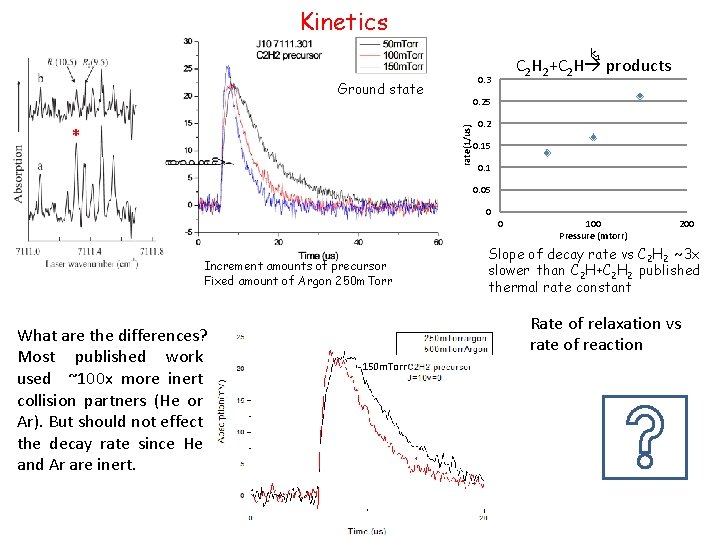
Kinetics k 1 0. 3 Ground state 0. 25 rate(1/us) * C 2 H 2+C 2 H products 0. 2 0. 15 0. 1 0. 05 0 0 Increment amounts of precursor Fixed amount of Argon 250 m. Torr What are the differences? Most published work used ~100 x more inert collision partners (He or Ar). But should not effect the decay rate since He and Ar are inert. 100 Pressure (mtorr) 200 Slope of decay rate vs C 2 H 2 ~3 x slower than C 2 H+C 2 H 2 published thermal rate constant Rate of relaxation vs rate of reaction 150 m. Torr

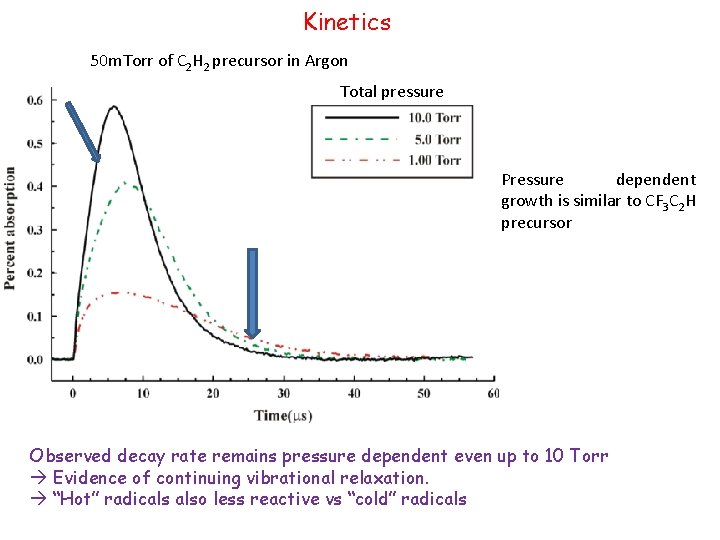
Kinetics 50 m. Torr of C 2 H 2 precursor in Argon Total pressure Pressure dependent growth is similar to CF 3 C 2 H precursor Observed decay rate remains pressure dependent even up to 10 Torr Evidence of continuing vibrational relaxation. “Hot” radicals also less reactive vs “cold” radicals

