Navigating the evolving therapeutic landscape in M 1








![Abiraterone vs Docetaxel (STAMPEDE post-hoc analysis) Failure-free survival [driven by PSA failure] SOC+AAP HR Abiraterone vs Docetaxel (STAMPEDE post-hoc analysis) Failure-free survival [driven by PSA failure] SOC+AAP HR](https://slidetodoc.com/presentation_image/801a6ddc4c2ec174f555b5c7c93b4b54/image-24.jpg)





- Slides: 35

Navigating the evolving therapeutic landscape in M 1 Prostate Cancer Karim Fizazi, MD, Ph. D Institut Gustave Roussy Villejuif, France

Disclosure Participation to advisory boards/honorarium for: Amgen, Astellas, Astrazeneca, Bayer, Curevac, Essa, Janssen, MSD, Orion, Sanofi

Incidence of de novo metastatic prostate cancer 5 -30% <5% 60% Wu JN, Cancer 2014; 120: 818 -823

Who Dies of Prostate Cancer? 44% of deaths Upfront localized cancer Metastases De novo metastases Death 56% of deaths Patrikidou A. Prostate Cancer Prostatic Dis. 2014; 17: 348 -352.

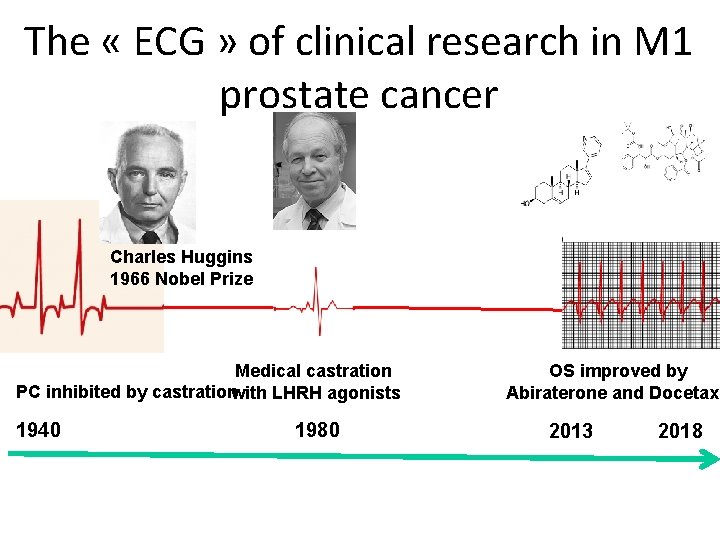
The « ECG » of clinical research in M 1 prostate cancer Charles Huggins 1966 Nobel Prize Medical castration PC inhibited by castrationwith LHRH agonists 1940 1980 OS improved by Abiraterone and Docetaxe 2013 2018

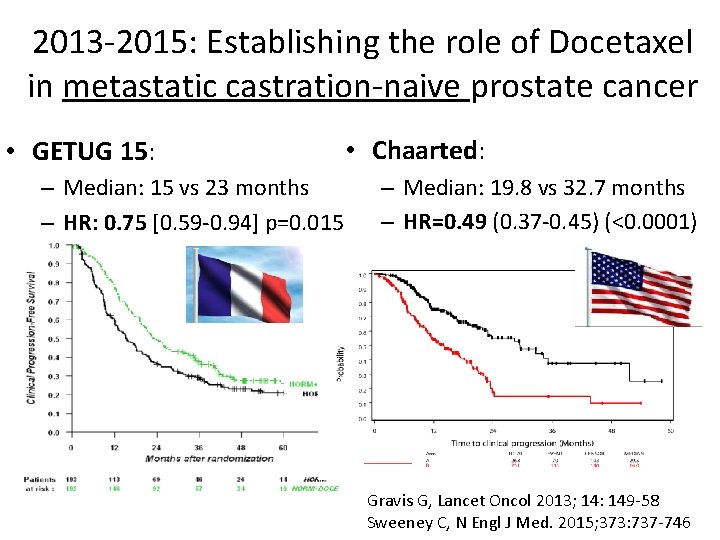
2013 -2015: Establishing the role of Docetaxel in metastatic castration-naive prostate cancer • GETUG 15: – Median: 15 vs 23 months – HR: 0. 75 [0. 59 -0. 94] p=0. 015 • Chaarted: – Median: 19. 8 vs 32. 7 months – HR=0. 49 (0. 37 -0. 45) (<0. 0001) Gravis G, Lancet Oncol 2013; 14: 149 -58 Sweeney C, N Engl J Med. 2015; 373: 737 -746

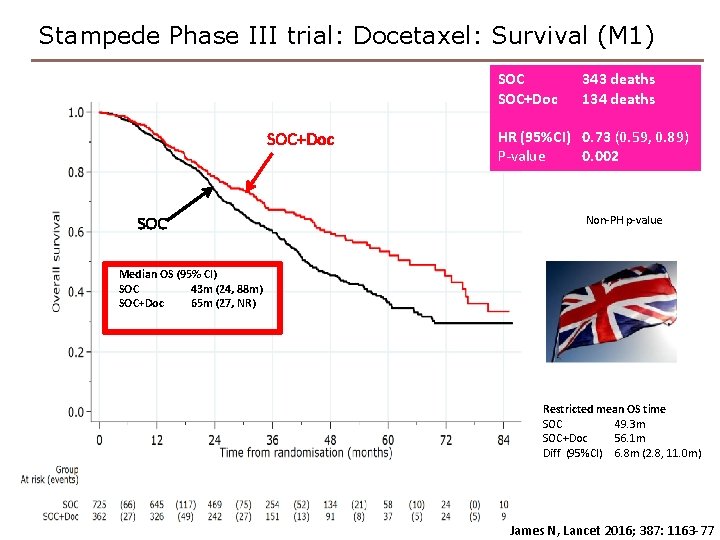
Stampede Phase III trial: Docetaxel: Survival (M 1) SOC+Doc SOC 343 deaths 134 deaths HR (95%CI) 0. 73 (0. 59, 0. 89) P-value 0. 002 Non-PH p-value Median OS (95% CI) SOC 43 m (24, 88 m) SOC+Doc 65 m (27, NR) Restricted mean OS time SOC 49. 3 m SOC+Doc 56. 1 m Diff (95%CI) 6. 8 m (2. 8, 11. 0 m) James N, Lancet 2016; 387: 1163 -77

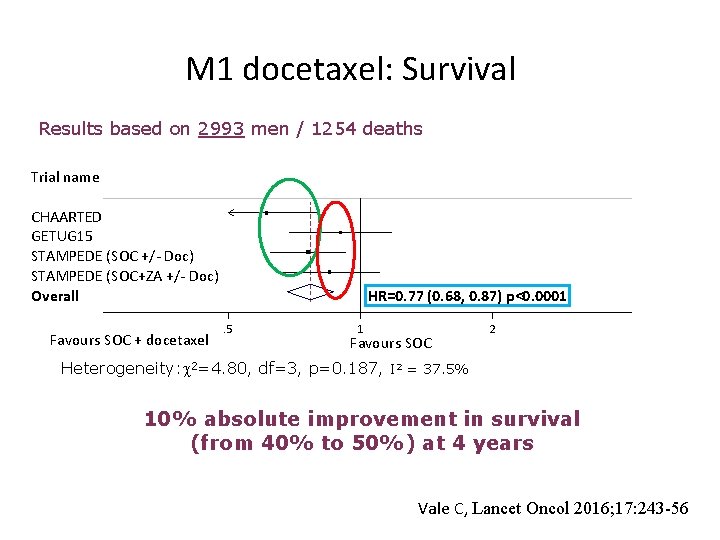
M 1 docetaxel: Survival Results based on 2993 men / 1254 deaths Trial name CHAARTED GETUG 15 STAMPEDE (SOC +/- Doc) STAMPEDE (SOC+ZA +/- Doc) Overall Favours SOC + docetaxel HR=0. 77 (0. 68, 0. 87) p<0. 0001. 5 1 Favours SOC 2 Heterogeneity: 2=4. 80, df=3, p=0. 187, I 2 = 37. 5% 10% absolute improvement in survival (from 40% to 50%) at 4 years Vale C, Lancet Oncol 2016; 17: 243 -56

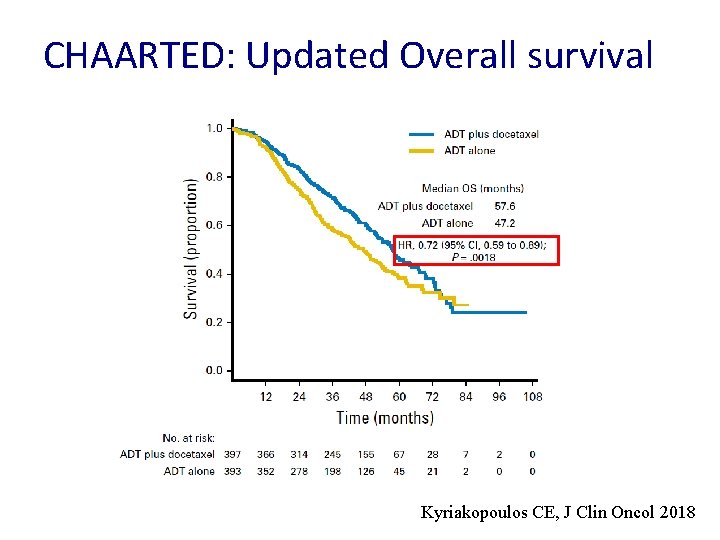
CHAARTED: Updated Overall survival Kyriakopoulos CE, J Clin Oncol 2018

Assessing prognosis in M 1 LATITUDE Low risk Max 1 poor-risk criterion High risk At least 2 risk criteria: - Gleason ≥ 8 - ≥ 3 bone mets - Visceral mets CHAARTED Low volume High volume No poor-risk criteria - ≥ 4 bone mets and - ≥ 1 beyond axial skeleton or - Visceral mets Sweeney C, N Engl J Med 2015; 373: 737 -46 Fizazi K, N Eng J Med 2017; 377: 352 -60

Assessing prognosis in M 1 OLIGO-METASTASES, GS=7 MULTIPLE METASTASES, GS≥ 8 Sweeney C, N Engl J Med 2015; 373: 737 Fizazi K, N Eng J Med 2017; 377: 352 -60

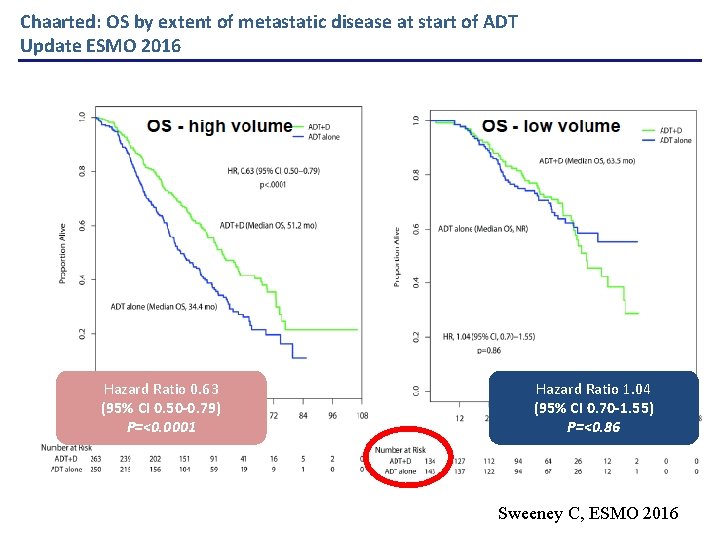
Chaarted: OS by extent of metastatic disease at start of ADT Update ESMO 2016 Hazard Ratio 0. 63 (95% CI 0. 50 -0. 79) P=<0. 0001 Hazard Ratio 1. 04 (95% CI 0. 70 -1. 55) P=<0. 86 Sweeney C, ESMO 2016

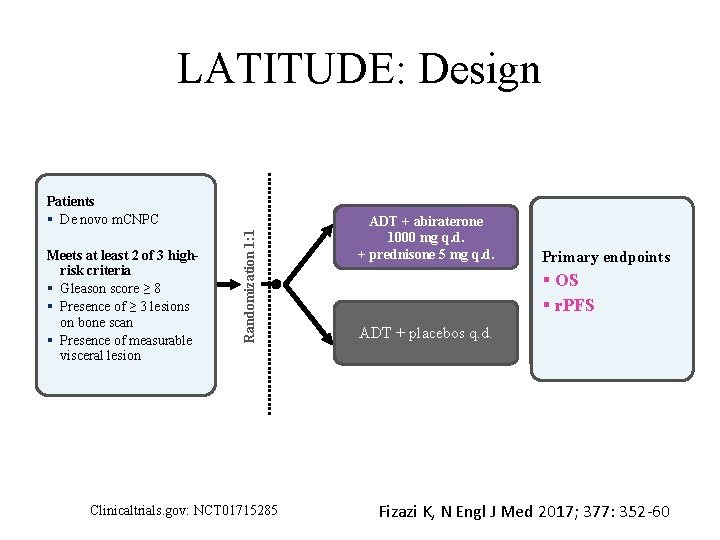
LATITUDE: Design Meets at least 2 of 3 highrisk criteria § Gleason score ≥ 8 § Presence of ≥ 3 lesions on bone scan § Presence of measurable visceral lesion Randomization 1: 1 Patients § De novo m. CNPC Clinicaltrials. gov: NCT 01715285 ADT + abiraterone Abiraterone 1000 mg QD + Prednisone mg QD 1000 mg 5 q. d. + ADT + prednisone 5 mg q. d. Placebo 1000 mg QD + Placebo 5 mg QDq. d. ADT + placebos + ADT Efficacy endpoints Primary: endpoints • Primary OS • r. PFS Secondary: • Time to next skeletal-related event • Time to PSA progression § OS § r. PFS Fizazi K, N Engl J Med 2017; 377: 352 -60

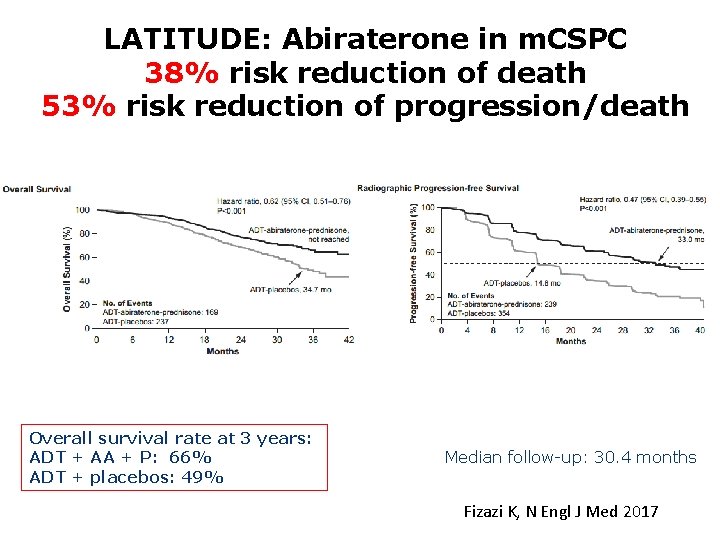
LATITUDE: Abiraterone in m. CSPC 38% risk reduction of death 53% risk reduction of progression/death Overall survival rate at 3 years: ADT + AA + P: 66% ADT + placebos: 49% Median follow-up: 30. 4 months Fizazi K, N Engl J Med 2017 19

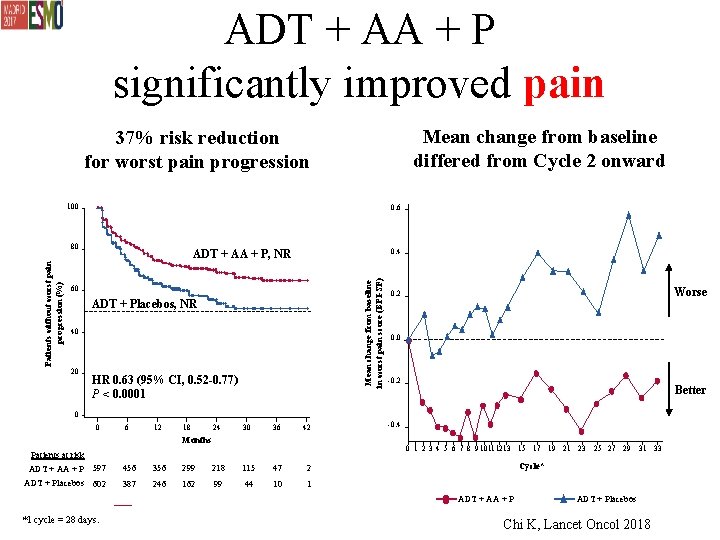
ADT + AA + P significantly improved pain Mean change from baseline differed from Cycle 2 onward 37% risk reduction for worst pain progression 100 0. 6 0. 4 ADT + AA + P, NR Mean change from baseline in worst pain score (BPI-SF) Patients without worst pain progression (%) 80 60 ADT + Placebos, NR 40 20 HR 0. 63 (95% CI, 0. 52 -0. 77) P < 0. 0001 Worse 0. 2 0. 0 -0. 2 Better 0 0 6 12 18 24 30 36 42 Months -0. 4 0 1 2 3 4 5 6 7 8 9 10 11 1213 15 17 19 21 23 25 27 29 31 33 Patients at risk ADT + AA + P 597 456 356 299 218 115 47 2 ADT + Placebos 602 387 246 162 99 44 10 1 Cycle* ADT + AA + P *1 cycle = 28 days. ADT + Placebos Chi K, Lancet Oncol 2018

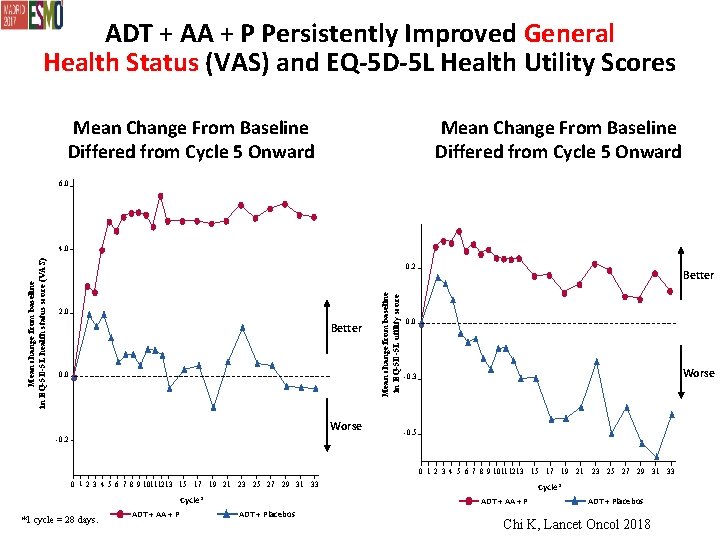
ADT + AA + P Persistently Improved General Health Status (VAS) and EQ-5 D-5 L Health Utility Scores Mean Change From Baseline Differed from Cycle 5 Onward 6. 0 0. 2 2. 0 Better 0. 0 Worse -0. 2 Mean change from baseline in EQ-5 D-5 L utility score Mean change from baseline in EQ-5 D-5 L health status score (VAS) 4. 0 Better 0. 0 Worse -0. 3 -0. 5 0 1 2 3 4 5 6 7 8 9 1011 12 13 15 17 19 21 23 25 27 29 31 33 0 1 2 3 4 5 6 7 8 9 1011 1213 15 17 19 21 23 25 27 29 31 33 Cycle* *1 cycle = 28 days. ADT + AA + P Cycle* ADT + AA + P ADT + Placebos Chi K, Lancet Oncol 2018

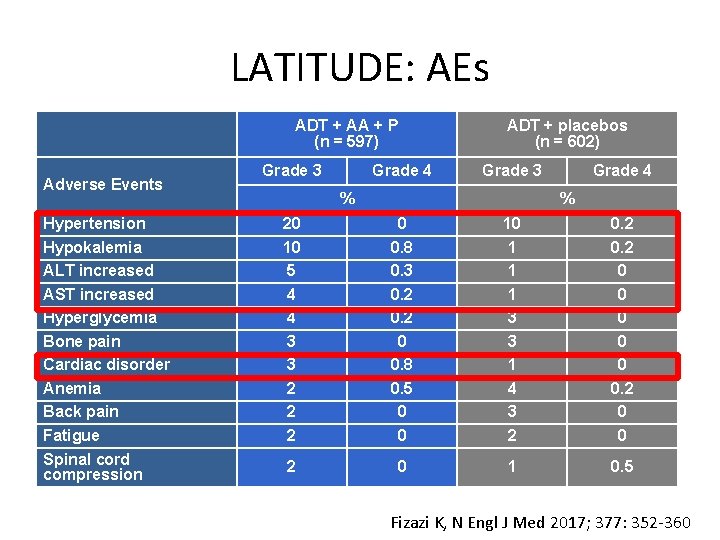
LATITUDE: AEs ADT + AA + P (n = 597) Adverse Events Hypertension Hypokalemia ALT increased AST increased Hyperglycemia Bone pain Cardiac disorder Anemia Back pain Fatigue Spinal cord compression Grade 3 Grade 4 ADT + placebos (n = 602) Grade 3 % Grade 4 % 20 10 5 4 4 3 3 2 2 2 0 0. 8 0. 3 0. 2 0 0. 8 0. 5 0 0 10 1 1 1 3 3 1 4 3 2 0 0 2 0 1 0. 5 Fizazi K, N Engl J Med 2017; 377: 352 -360

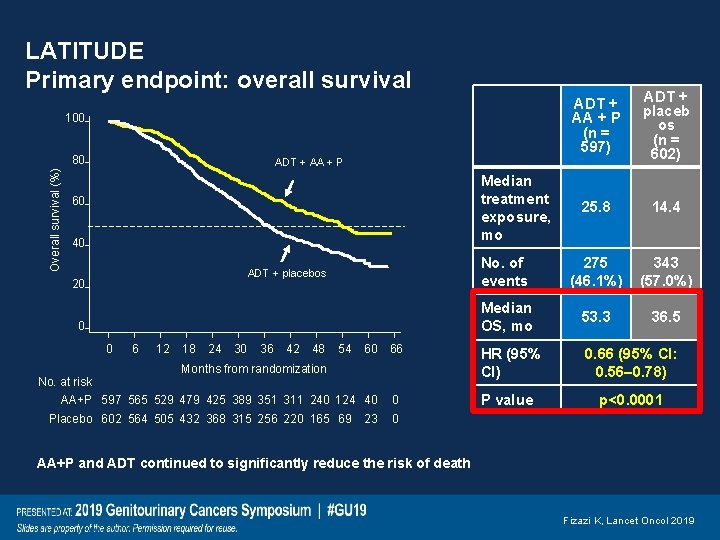
LATITUDE Primary endpoint: overall survival ADT + AA + P (n = 597) ADT + placeb os (n = 602) 25. 8 14. 4 No. of events 275 (46. 1%) 343 (57. 0%) Median OS, mo 53. 3 36. 5 66 HR (95% CI) 0. 66 (95% CI: 0. 56 0. 78) 0 P value 100 Overall survival (%) 80 ADT + AA + P Median treatment exposure, mo 60 40 ADT + placebos 20 0 0 No. at risk 6 12 18 24 30 36 42 48 54 60 Months from randomization AA+P 597 565 529 479 425 389 351 311 240 124 40 Placebo 602 564 505 432 368 315 256 220 165 69 23 p<0. 0001 0 AA+P and ADT continued to significantly reduce the risk of death Fizazi K, Lancet Oncol 2019

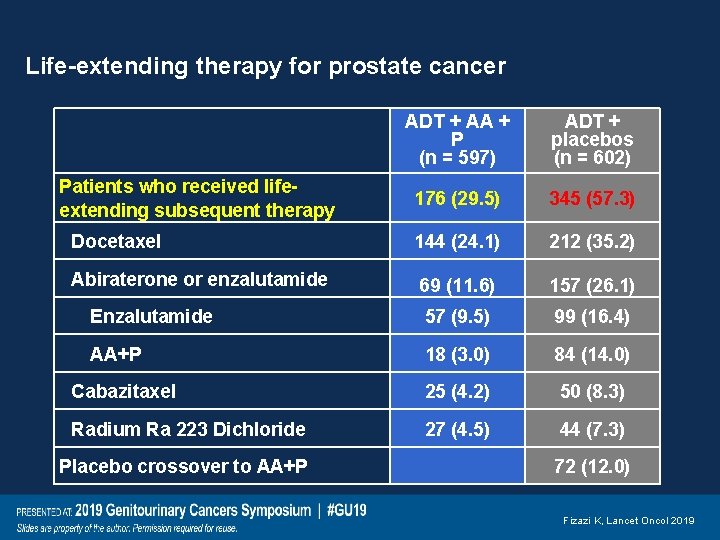
Life-extending therapy for prostate cancer ADT + AA + P (n = 597) ADT + placebos (n = 602) 176 (29. 5) 345 (57. 3) Docetaxel 144 (24. 1) 212 (35. 2) Abiraterone or enzalutamide 69 (11. 6) 157 (26. 1) Enzalutamide 57 (9. 5) 99 (16. 4) AA+P 18 (3. 0) 84 (14. 0) Cabazitaxel 25 (4. 2) 50 (8. 3) Radium Ra 223 Dichloride 27 (4. 5) 44 (7. 3) Placebo crossover to AA+P 72 (12. 0) Patients who received lifeextending subsequent therapy Fizazi K, Lancet Oncol 2019

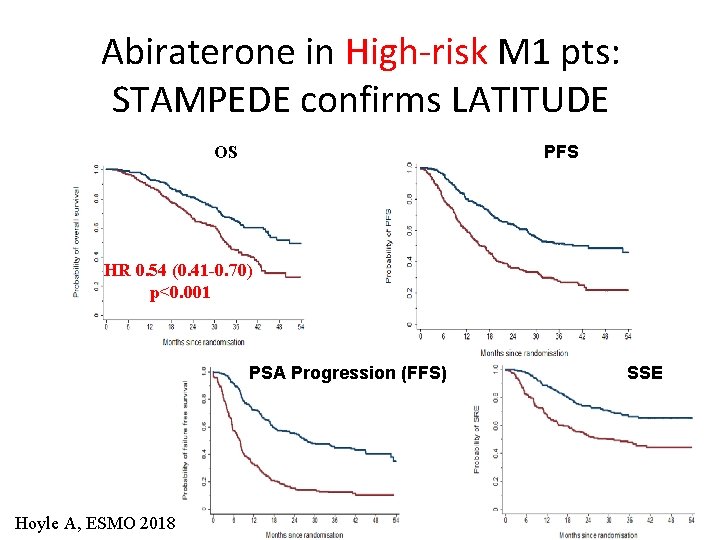
Abiraterone in High-risk M 1 pts: STAMPEDE confirms LATITUDE PFS OS HR 0. 54 (0. 41 -0. 70) p<0. 001 PSA Progression (FFS) Hoyle A, ESMO 2018 SSE

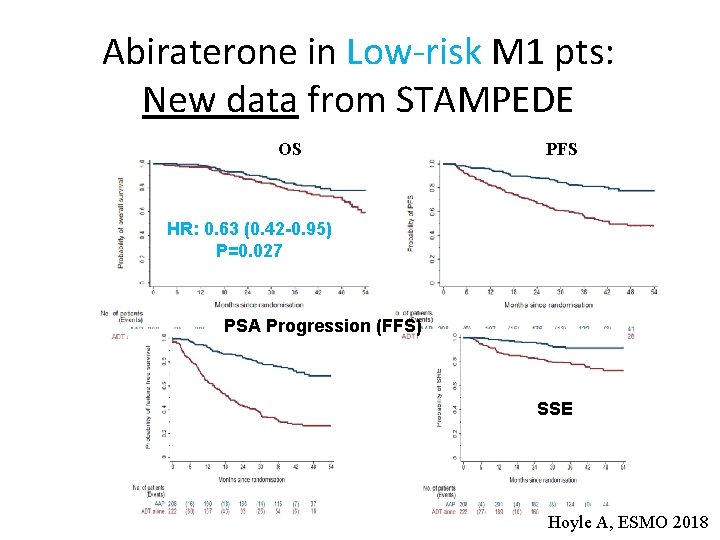
Abiraterone in Low-risk M 1 pts: New data from STAMPEDE OS PFS HR: 0. 63 (0. 42 -0. 95) P=0. 027 PSA Progression (FFS) SSE Hoyle A, ESMO 2018

Abiraterone vs Docetaxel (OS, indirect comparison) Feyerabend S, Eur J Cancer 2018; 103: 78 -87 Rydzewska L, Eur J Cancer 2017; 84: 88 -101

PRO: Indirect comparison of LATITUDE and CHAARTED Patient function (FACT-P) Pain (BPI) Feyerabend S, et al. Eur J Cancer 2018; 103: 78 -87
![Abiraterone vs Docetaxel STAMPEDE posthoc analysis Failurefree survival driven by PSA failure SOCAAP HR Abiraterone vs Docetaxel (STAMPEDE post-hoc analysis) Failure-free survival [driven by PSA failure] SOC+AAP HR](https://slidetodoc.com/presentation_image/801a6ddc4c2ec174f555b5c7c93b4b54/image-24.jpg)
Abiraterone vs Docetaxel (STAMPEDE post-hoc analysis) Failure-free survival [driven by PSA failure] SOC+AAP HR (95%CI) P-val SOC+Doc. P Interactn test 0. 17 M 1 0. 56 (0. 42 to 0. 75) <0. 001 Key: HR<1 favours SOC+AAP HR>1 favours SOC+Doc. P Interactn = test for interaction (heterogeneity of treatment effect) Sydes M, Ann Oncol 2018; 29: 1235 -48

PEACE-1: European Phase III Trial in de novo Metastatic Prostate Cancer (revised design) SOC • Patients with newly diagnosed (castration -naïve) metastatic Ca. P • 1156 pts planned R A N D O M I Z E D SOC + Abiraterone 1000 mg Prednisone 5 mg BID SOC + Co-primary endpoints: OS and PFS (HR: 0. 75) Local radiotherapy SOC + Local radiotherapy + Abiraterone-Pred Standard of Care (SOC)= Androgen deprivation therapy (ADT) +/- docetaxel (Stratification) Clinical. Trials. gov. Identifier: NCT 01957436. Study sponsor: Unicancer

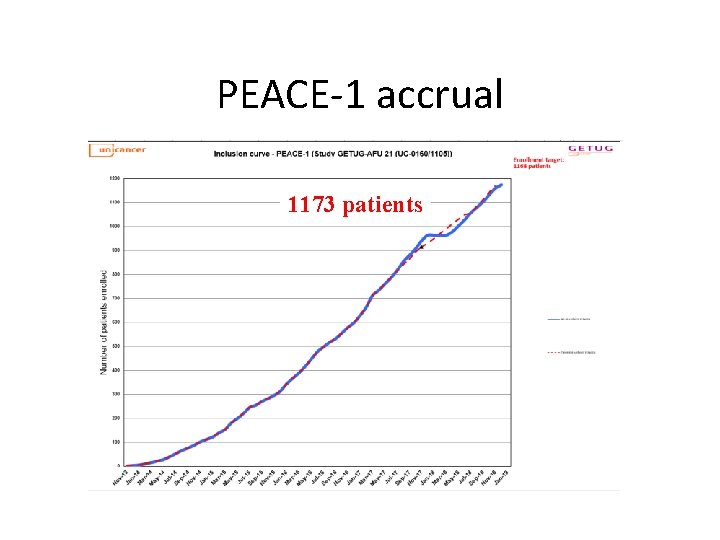
PEACE-1 accrual 1173 patients

Phase 3 Ongoing Combination Therapy Trials Study Identifier Arms Pts (N) Primary End Point Status LATITUDE NCT 01715285 ADT ± AA 1209 r. PFS, OS Ongoing STAMPEDE (Arm G) NCT 00268476 ADT ± AA 1800 OS Ongoing PEACE-1 NCT 01957436 ADT ± DOC vs ADT + AA ± DOC (± local RT) 1173 PFS, OS Ongoing STAMPEDE (Arm J) NCT 00268476 ADT ± AA + ENZ 1800 OS Ongoing SWOG-1216 NCT 01809691 ADT + TAK-700 vs ADT + BIC 1304 OS Recruiting ENZAMET NCT 02446405 ADT + ENZ vs ADT + antiandrogen 1100 OS Ongoing TITAN NCT 02489318 ADT ± APA 1000 r. PFS, OS Ongoing ARCHES NCT 02677896 ADT ± ENZ 1100 r. PFS Ongoing ARASENS NCT 02799602 ADT + DOC ± ODM-201 1300 OS Ongoing 36

Enzalutamide in M 1: ARCHES primary endpoint: r. PFS CI, confidence interval; HR, hazard ratio; NR, not reached. Armstrong AJ, et al. Oral presentation at ASCO GU 2019; abstract 687.

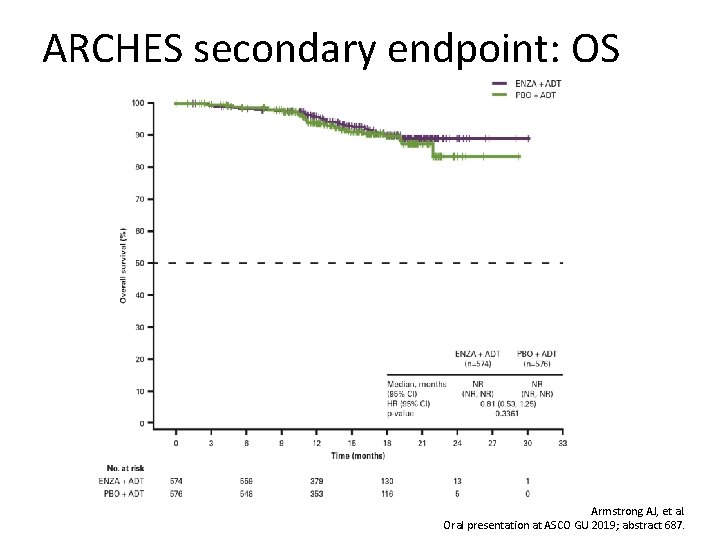
ARCHES secondary endpoint: OS Armstrong AJ, et al. Oral presentation at ASCO GU 2019; abstract 687.

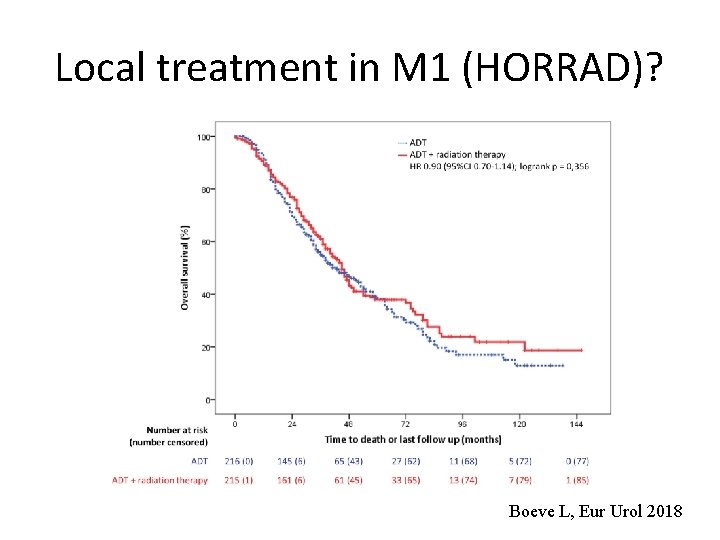
Local treatment in M 1 (HORRAD)? Boeve L, Eur Urol 2018

Overall survival: prostate radiotherapy in M 1 (STAMPEDE) Low burden High burden SOC+RT SOC SOC+RT HR: 0. 68 (95% CI 0. 52 -0. 90); p=0. 007 3 year OS (%): SOC = 73% SOC+RT = 81% HR: 1. 07 (95% CI 0. 90 -1. 28); p=0. 420 3 year OS (%): SOC = 54% SOC+RT = 53% Parker C, Lancet 2018

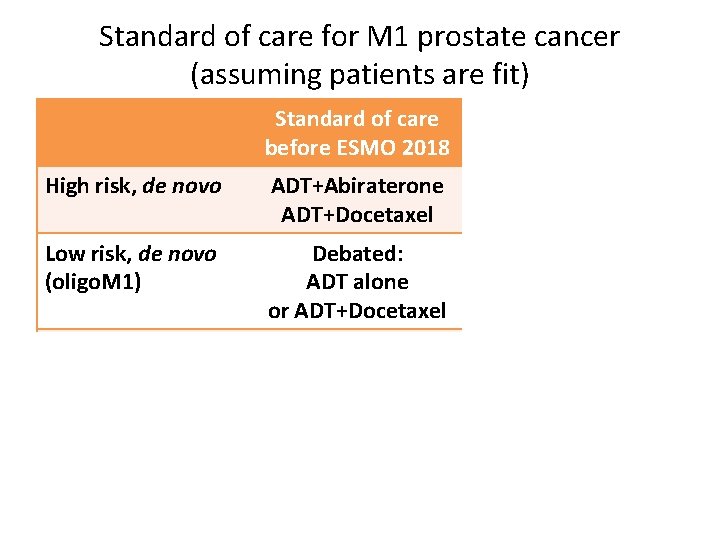
Standard of care for M 1 prostate cancer (assuming patients are fit) Standard of care before ESMO 2018 Standard of care after ESMO 2018 High risk, de novo ADT+Abiraterone ADT+Docetaxel Low risk, de novo (oligo. M 1) Debated: ADT+ Abiraterone ADT alone RXT of the prostate or ADT+Docetaxel ? Previous local Tx, low risk ? ? Previous local Tx, high risk ? ? Metastases detected on PET ? ?

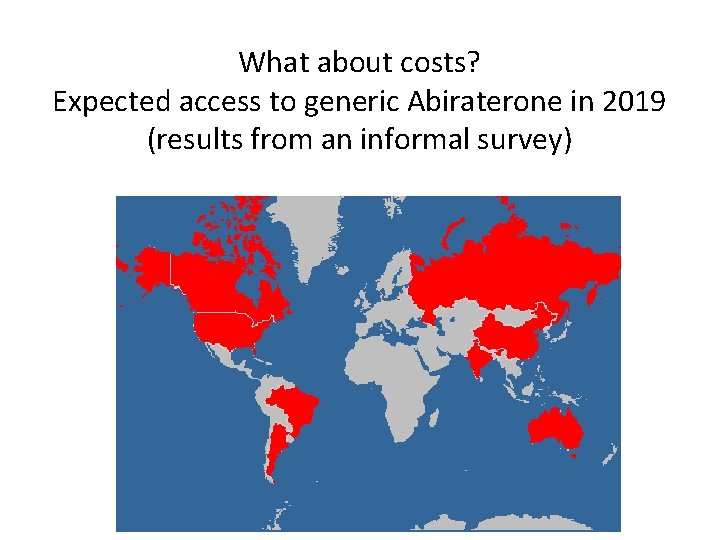
What about costs? Expected access to generic Abiraterone in 2019 (results from an informal survey)

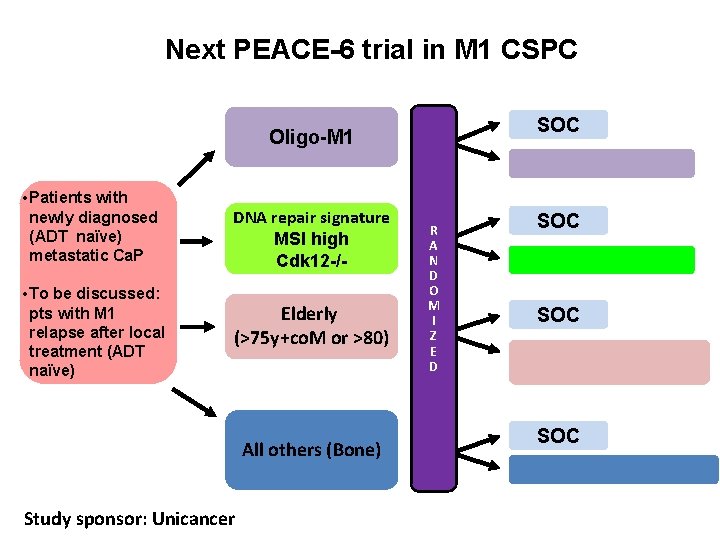
Next PEACE-6 trial in M 1 CSPC SOC Oligo-M 1 • Patients with newly diagnosed (ADT naïve) metastatic Ca. P • To be discussed: pts with M 1 relapse after local treatment (ADT naïve) DNA repair signature MSI high Cdk 12 -/- Elderly (>75 y+co. M or >80) All others (Bone) Study sponsor: Unicancer R A N D O M I Z E D SOC SOC

Conclusion • Current systemic treatment for M 1 patients: – ADT + Abiraterone – ADT + Docetaxel – ADT alone for frail/very elderly patients? • RXT to prostate for oligo-M 1 • Triplet? (ADT+Abiraterone+Docetaxel): PEACE-1 • Next-generation trial stratified on clinical/molecular parameters: PEACE-6
 Digital landscape model
Digital landscape model Evolving
Evolving Evolving design
Evolving design A framework for clustering evolving data streams
A framework for clustering evolving data streams Key evolving signature
Key evolving signature Navigating gdpr compliance on aws
Navigating gdpr compliance on aws Navigating the body muscular system #2
Navigating the body muscular system #2 Navigating the art world
Navigating the art world Cadet rule in navigation
Cadet rule in navigation Mks aws
Mks aws Applications of trigonometry in navigation
Applications of trigonometry in navigation Lời thề hippocrates
Lời thề hippocrates Dạng đột biến một nhiễm là
Dạng đột biến một nhiễm là Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể độ dài liên kết
độ dài liên kết Môn thể thao bắt đầu bằng từ đua
Môn thể thao bắt đầu bằng từ đua Khi nào hổ mẹ dạy hổ con săn mồi
Khi nào hổ mẹ dạy hổ con săn mồi điện thế nghỉ
điện thế nghỉ Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ Một số thể thơ truyền thống
Một số thể thơ truyền thống Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Chó sói
Chó sói Thiếu nhi thế giới liên hoan
Thiếu nhi thế giới liên hoan Số nguyên tố là số gì
Số nguyên tố là số gì Fecboak
Fecboak Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư tọa độ 5x5
Mật thư tọa độ 5x5 Tư thế worm breton
Tư thế worm breton Bổ thể
Bổ thể ưu thế lai là gì
ưu thế lai là gì Tư thế ngồi viết
Tư thế ngồi viết