Natural Transmutation Aim Nuke 3 When an element



- Slides: 15

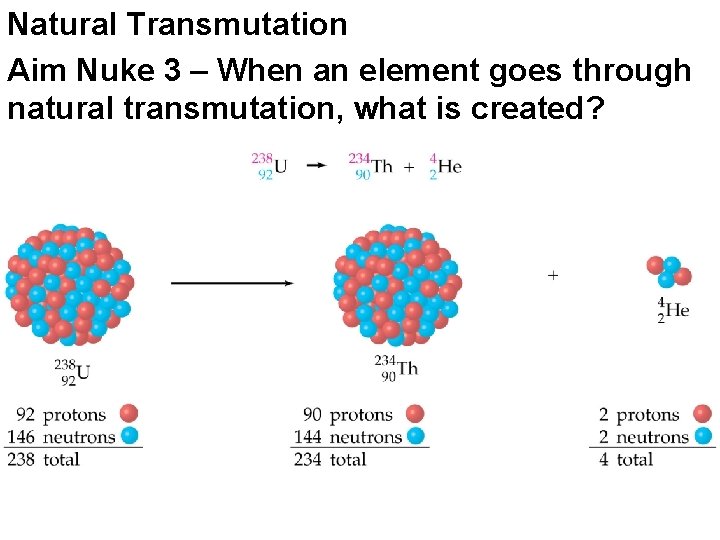
Natural Transmutation Aim Nuke 3 – When an element goes through natural transmutation, what is created?

Radiation Emissions When natural transmutation occurs A radioactive particle is released changing the radioisotope into a totally different element Conservation of Matter applies What you start with equals what you end up with The sum of the mass numbers and atomic numbers on the left of the reaction (reactants) equals the sum of the mass numbers and atomic numbers on the right (products)

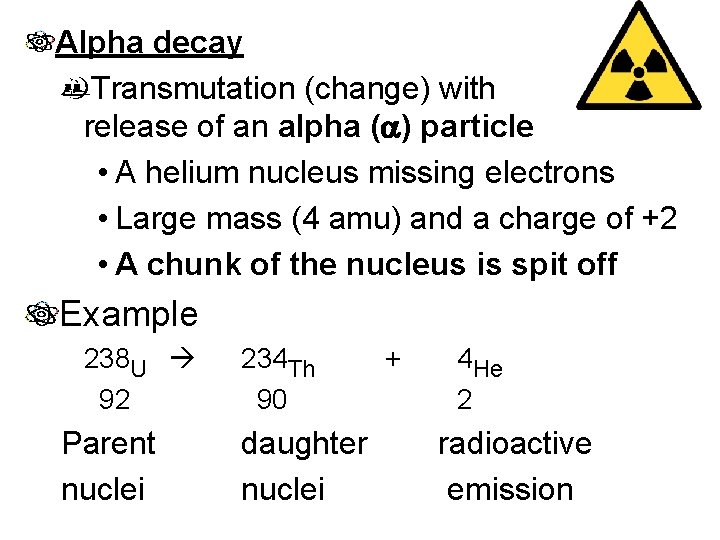
Alpha decay Transmutation (change) with release of an alpha (a) particle • A helium nucleus missing electrons • Large mass (4 amu) and a charge of +2 • A chunk of the nucleus is spit off Example 238 U 92 Parent nuclei 234 Th 90 daughter nuclei + 4 He 2 radioactive emission

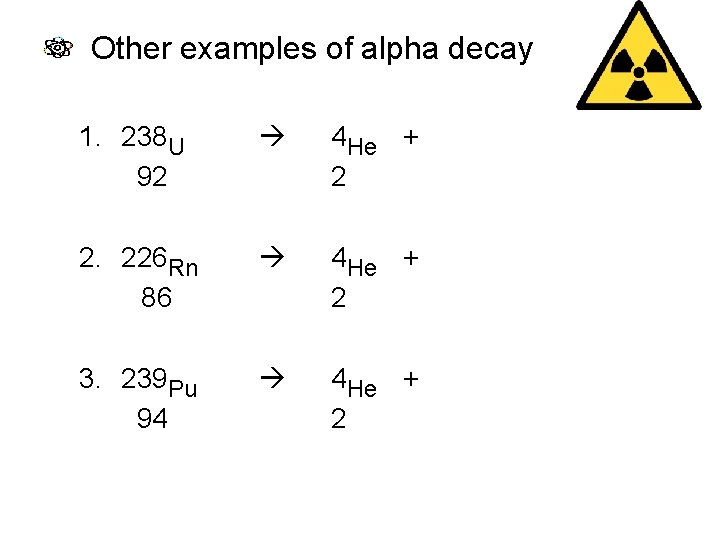
Other examples of alpha decay 1. 238 U 92 4 He + 234 Th 2 90 2. 226 Rn 86 4 He + 222 Po 2 84 3. 239 Pu 94 4 He + 235 U 2 92

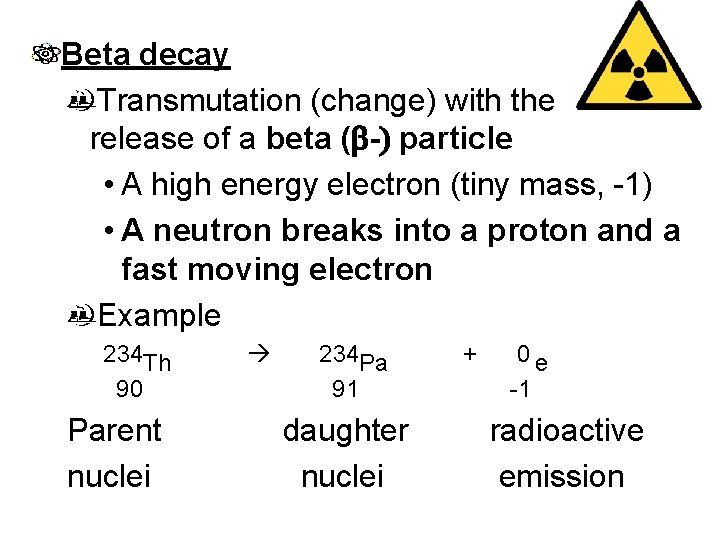
Beta decay Transmutation (change) with the release of a beta (b-) particle • A high energy electron (tiny mass, -1) • A neutron breaks into a proton and a fast moving electron Example 234 Th 90 Parent nuclei 234 Pa 91 daughter nuclei + 0 e -1 radioactive emission

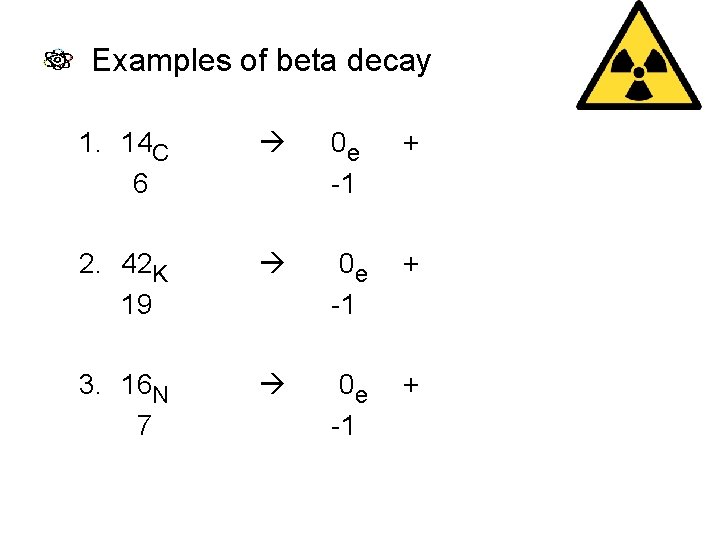
Examples of beta decay 1. 14 C 6 0 e -1 + 14 N 7 2. 42 K 19 0 e -1 + 42 Ca 20 3. 16 N 7 0 e -1 + 16 O 8

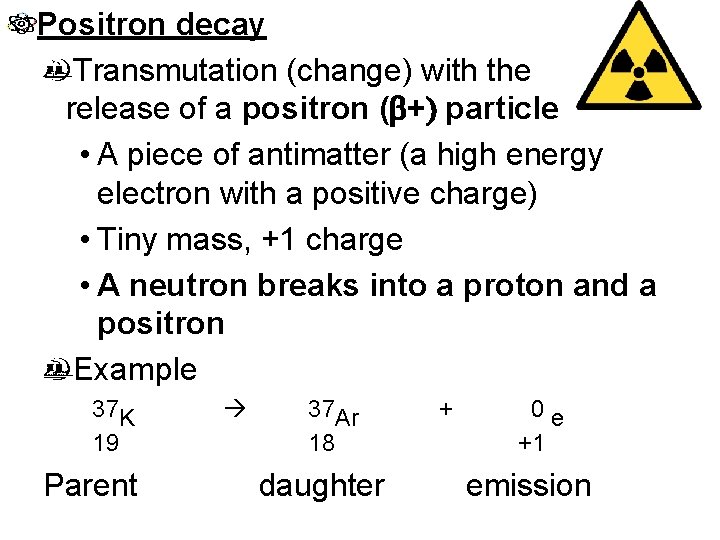
Positron decay Transmutation (change) with the release of a positron (b+) particle • A piece of antimatter (a high energy electron with a positive charge) • Tiny mass, +1 charge • A neutron breaks into a proton and a positron Example 37 K 19 Parent 37 Ar 18 daughter + 0 e +1 emission

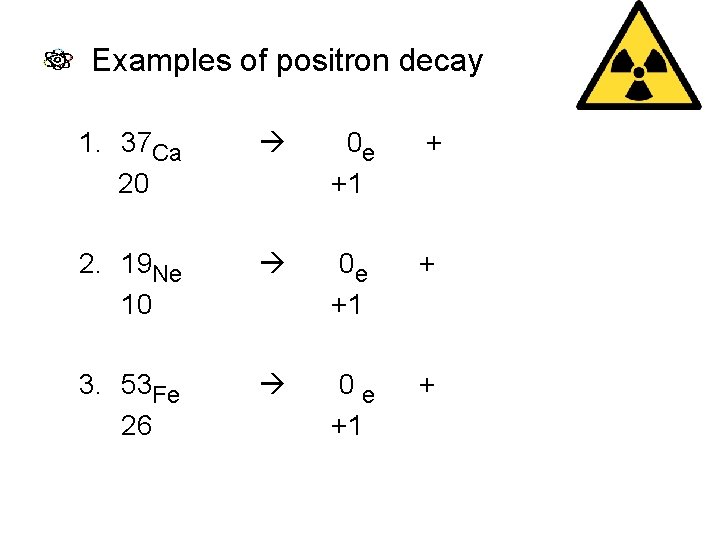
Examples of positron decay 1. 37 Ca 20 0 e +1 + 37 K 19 2. 19 Ne 10 0 e +1 + 19 F 9 3. 53 Fe 26 0 e +1 + 53 Mn 25

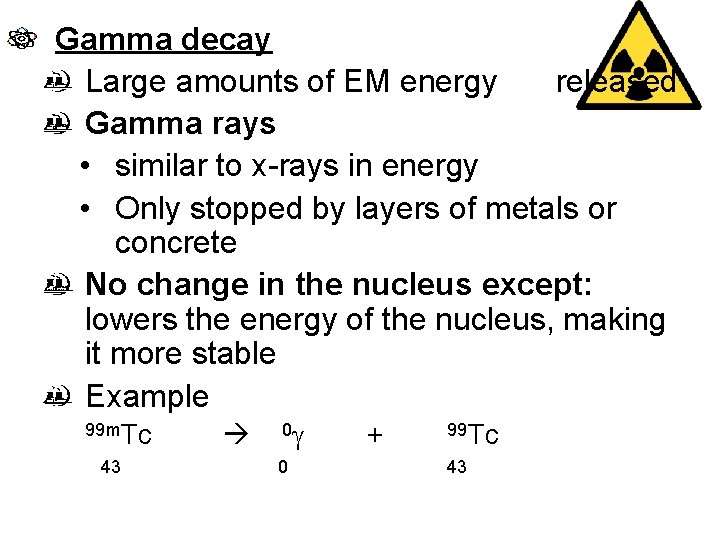
Gamma decay Large amounts of EM energy released Gamma rays • similar to x-rays in energy • Only stopped by layers of metals or concrete No change in the nucleus except: lowers the energy of the nucleus, making it more stable Example 99 m. Tc 43 0 g 0 + 99 Tc 43

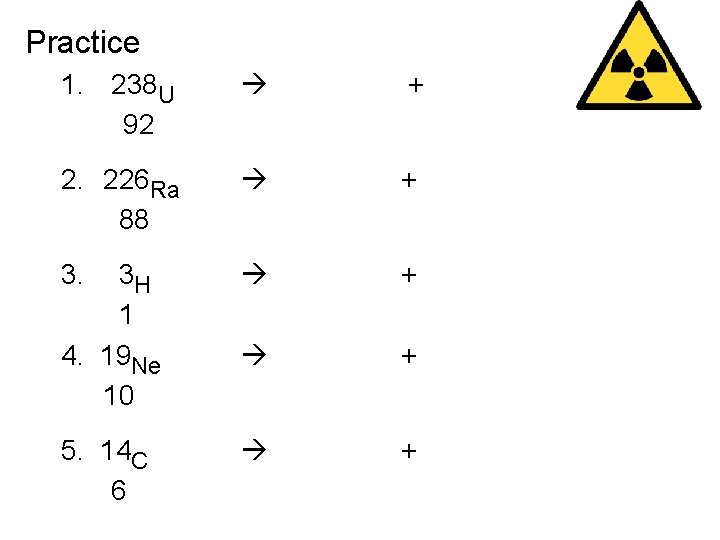
Practice 1. 238 U 92 4 He 2 + 234 Th 90 2. 226 Ra 88 4 He 2 + 222 Rn 86 3. 3 H 1 4. 19 Ne 10 0 e -1 0 e +1 + 3 He 2 19 F 9 5. 14 C 6 0 e -1 + + 14 N 7

Aim Nuke 3 – What is artificial transmutation?

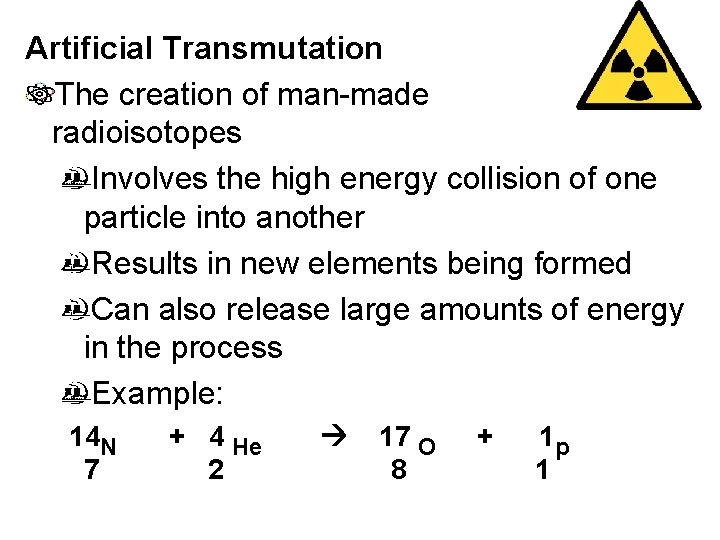
Artificial Transmutation The creation of man-made radioisotopes Involves the high energy collision of one particle into another Results in new elements being formed Can also release large amounts of energy in the process Example: 14 N 7 + 4 He 2 17 O 8 + 1 p 1

Artificial Transmutation Particle accelerators Also known as atom smashers They use magnetic and electric fields to accelerate or “push” charged particles This allows particles to crash together to gain enough energy to overcome forces in the nucleus Analogy shoot a bullet at a clock it shatters The pieces that fly out tell us what’s in it

Other examples of artificial transmutation Example 1 - What is the nuclear equation for the bombardment of nitrogen-14 nuclei with alpha particles to produce artificially transmuted oxygen-17? 14 N + 4 He 7 2 18 O + 0 e 8 +1

Other examples of artificial transmutation Ex 2 – aluminum-27 is bombarded with alpha particles to produce radioactive phosphorus-30 What does the nuclear equation look like? 27 Al + 4 He 13 2 30 P + 1 n 15 0