Natural Health Products Program Logic Model Reach Academia
- Slides: 1

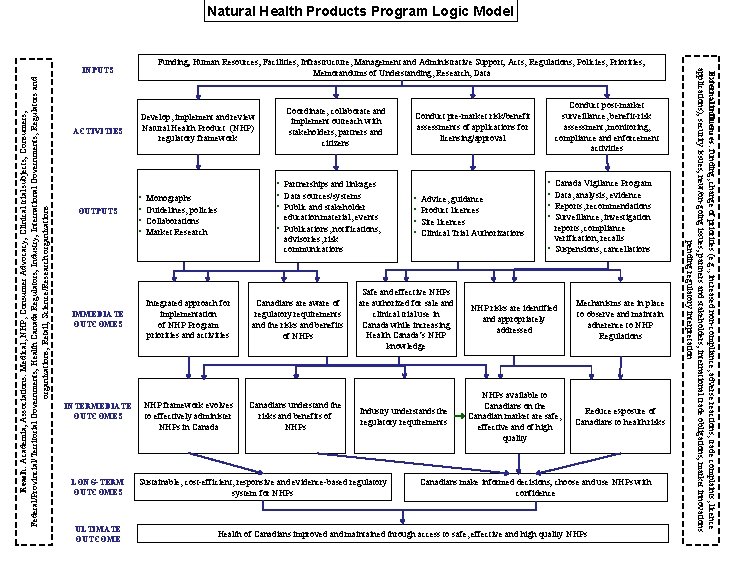
Natural Health Products Program Logic Model Reach: Academia; Associations: Medical, NHP, Consumer Advocacy; Clinical trial subjects; Consumers; Federal/Provincial/Territorial Governments; Health Canada Regulators; Industry; International Governments, Regulators and organizations; Retail; Science/Research organizations ACTIVITIES OUTPUTS IMMEDIATE OUTCOMES INTERMEDIATE OUTCOMES LONG-TERM OUTCOMES ULTIMATE OUTCOME Develop, implement and review Natural Health Product (NHP) regulatory framework • • Coordinate, collaborate and implement outreach with stakeholders, partners and citizens • Partnerships and linkages • Data sources/systems • Public and stakeholder Monographs Guidelines, policies Collaborations Market Research education material, events • Publications, notifications, advisories, risk communications Integrated approach for implementation of NHP Program priorities and activities Canadians are aware of regulatory requirements and the risks and benefits of NHPs NHP framework evolves to effectively administer NHPs in Canada Canadians understand the risks and benefits of NHPs Conduct post-market surveillance, benefit-risk assessment, monitoring, compliance and enforcement activities Conduct pre-market risk/benefit assessments of applications for licensing/approval • • Advice, guidance Product licences Site licences Clinical Trial Authorizations • • Canada Vigilance Program Data, analysis, evidence Reports, recommendations Surveillance, investigation reports, compliance verification, recalls • Suspensions, cancellations Safe and effective NHPs are authorized for sale and clinical trial use in Canada while increasing Health Canada’s NHP knowledge NHP risks are identified and appropriately addressed Mechanisms are in place to observe and maintain adherence to NHP Regulations Industry understands the regulatory requirements NHPs available to Canadians on the Canadian market are safe, effective and of high quality Reduce exposure of Canadians to health risks Sustainable, cost-efficient, responsive and evidence-based regulatory system for NHPs Canadians make informed decisions; choose and use NHPs with confidence Health of Canadians improved and maintained through access to safe, effective and high quality NHPs External influences: funding, change of priorities (e. g. , increased non-compliance, adverse reactions, trade complaints, licence applications); security issues; new/on-going issues; partners and stakeholders; international trade obligations; market innovations pending regulatory interpretation Funding; Human Resources; Facilities, Infrastructure, Management and Administrative Support; Acts, Regulations, Policies, Priorities; Memorandums of Understanding, Research, Data INPUTS