NATIONAL LUNG HOSPITAL ONCOLOGY DEPARTMENT NGUYEN THANH DUONG

















- Slides: 33

NATIONAL LUNG HOSPITAL ONCOLOGY DEPARTMENT NGUYEN THANH DUONG MD EVALUATE THE RESULTS OF TREATMENT NON SMALL CELL LUNG CANCER IN STAGE IV OF GEMCITABINE & CISPLATIN REGIMEN AT THE NATIONAL LUNG HOSPITAL Scientific Guide teacher Asso. Pro LE TRUNG THO

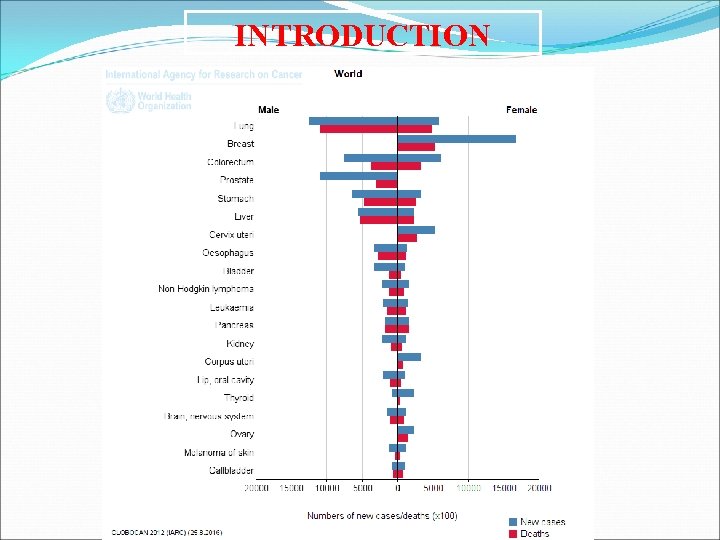
INTRODUCTION - Lung cancer is the most malignancy disease in the worldwide - + Estimated total 1. 8 million new cases each year. + About 1. 6 million deaths - In Viet Nam, according Globocan 2012: + 21865 new cases about 24. 4 % of total + 19559 deaths of lung cancer accounted for 21. 8%.

INTRODUCTION

INTRODUCTION - Lung cancer is a highly malignant, rapid progressive , early metastasis in many organs such as liver, bone, brain. - Diagnosis at late stage, can not be treated by surgery (about 70%). - Chemotherapy are used in the late stage - Gemcitabine is a remarkably effective chemotherapeutic agent for the treatment of late-stage, non-small cell lung cancer, when used in combination with cisplatin (or carboplatin) in clinical trials.

THE PURPOSE - In the Department of Oncology, Gemcitabine-Cisplatin regimen has also been used in the treatment of NSCLC since 2013 but no study has evaluated the efficacy of this regimen. Therefore, the research objectives of the project are:

THE AIM OF STUDY ØCommentary on some clinical and subclinical features of patients with stage IV non-small cell lung cancer in National Lung Hospital from 2013 to 2016. ØEstimation of response rate, overall survival and some toxicities of Gemcitabine-Cisplatin regimen.

LITERATURE REVIEW v. Etiology and related factors : - Tobacco: is the main cause of 90% of patients were diagnosed NSCLC related tobacco. Smokers are more 10 to 20 times risk likely to develop NSCLC. - Substances that do not involve tobacco : Arsen, Amiang, Nickel, Radon, Diesel smoke… - Relevant factors : + Age: Most patients are diagnosed with 35 to 75 years of age + Sex: Worldwide: male/female: 6: 1 + Peak 55 - 65 years old Viet Nam: 4/1 Other factors: family, diet, chronic lung diseases, socioeconomic.

LITERATURE REVIEW v Clinical symptoms : A. Chest: - Cough: dry cough, sputum cough, coughing up blood - Chest pain - Dysnea B. Symptoms due to the cancer mass pressing on adjacent structures - Hoarse-voiced, hiccup, gag - Superior vena cava syndrome - Pancoast – Tobias syndrome - Claude – Bernard – Horner syndrome - Pleural and pericardia infusion - Paraneoplastic syndrome C. Whole body: Fever, anorectic, weight loss D. Mestastasis symptoms: headeach(brain mestastasis), bone mestastasis, colic (hepa-mestastasis)…

LITERATURE REVIEW v Subclinical - Image analysation: Chest X_ray, CT Scanner, MRI, Spect, PET – CT, ultrasound - Tumour marker : CEA, Cyfra 21 -1 - Endoscopy: Bronchoscopy - Cytology: pleural fluid, pericardial fluid, bronchial fluid, … - Pathology: gold standard - EGFR mutation

OBJECTIVES AND RESEARCH METHODS v OBJECTIVES: v Inclusion criteria: - Diagnosis NSCLC stage IV ( AJCC 2010) - Kanofsky ≥ 70 - Do not have other cancers or severe or chronic illnesses - Do not receive prior chemotherapy or any other systemic therapy for advanced NSCLC - At least treatment 4 cycler Gemcitabine + Cisplatin - Written informed consent form and full clinical resource forms

OBJECTIVES AND RESEARCH METHODS Ø Exclusion criteria: - Not satisfied with any inclusion criteria. - Contraindications for chemotherapy - Target therapy concomitant - There are 2 cancers or no definable primary tumors in the lung

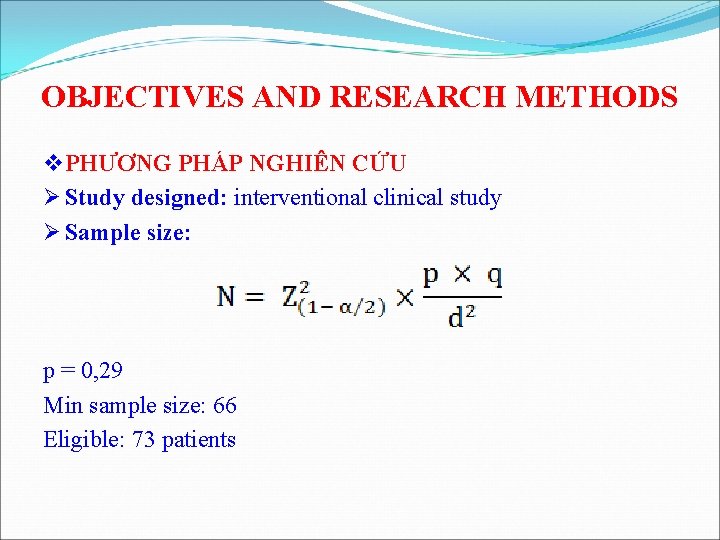
OBJECTIVES AND RESEARCH METHODS v PHƯƠNG PHÁP NGHIÊN CỨU Ø Study designed: interventional clinical study Ø Sample size: p = 0, 29 Min sample size: 66 Eligible: 73 patients

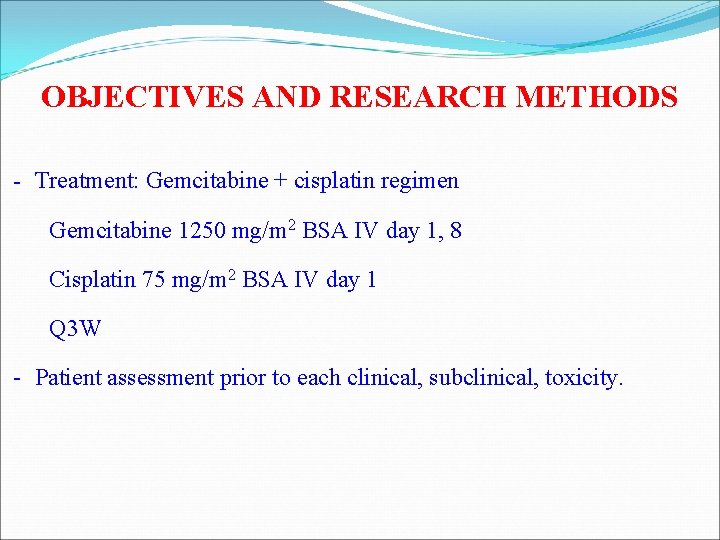
OBJECTIVES AND RESEARCH METHODS - Treatment: Gemcitabine + cisplatin regimen Gemcitabine 1250 mg/m 2 BSA IV day 1, 8 Cisplatin 75 mg/m 2 BSA IV day 1 Q 3 W - Patient assessment prior to each clinical, subclinical, toxicity.

RESULTS AND COMMENTS v Characteristics of the patient : High frequency in the age group 60 -69 (38. 4%) and age group 50 -59 (37%). The frequency of the group aged 50 -69 was 75. 4%, significantly higher than the othẻ age group with p <0. 005.

RESULTS AND COMMENTS Male/Female = 3 / 1 * Phan Le Thang (2001): male/female : 4, 1/1 * Hoang Trong Tung (2006): male/female: 4, 8/1

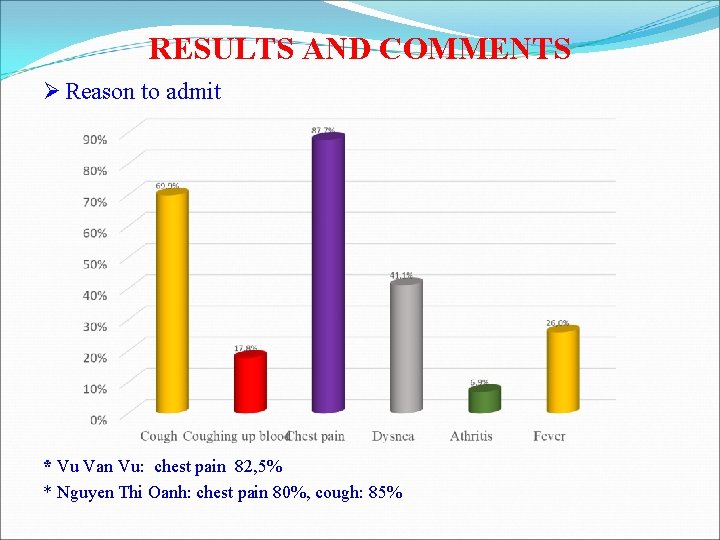
RESULTS AND COMMENTS Ø Reason to admit * Vu Van Vu: chest pain 82, 5% * Nguyen Thi Oanh: chest pain 80%, cough: 85%

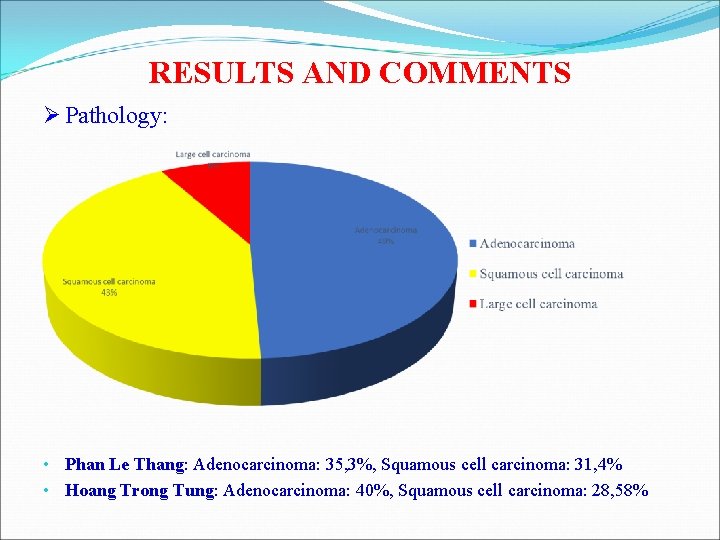
RESULTS AND COMMENTS Ø Pathology: • Phan Le Thang: Adenocarcinoma: 35, 3%, Squamous cell carcinoma: 31, 4% • Hoang Trong Tung: Adenocarcinoma: 40%, Squamous cell carcinoma: 28, 58%

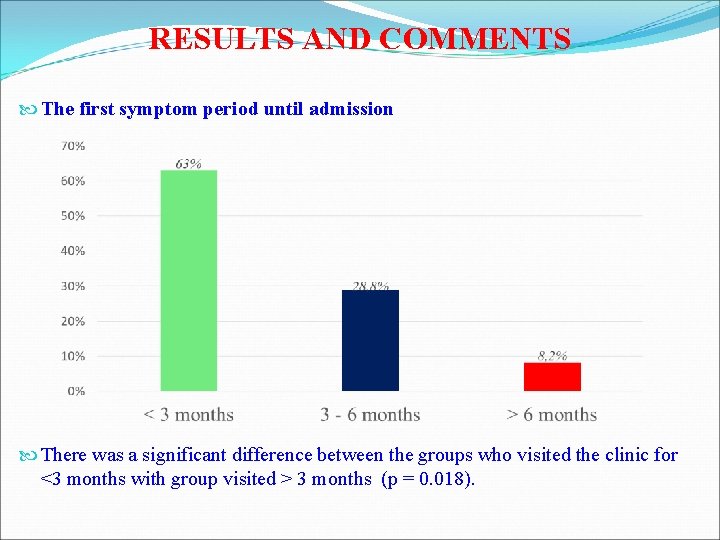
RESULTS AND COMMENTS The first symptom period until admission There was a significant difference between the groups who visited the clinic for <3 months with group visited > 3 months (p = 0. 018).

RESULTS AND COMMENTS ØCTscanner: Situation Central Periphery No. Rate % No. Rate% Right Lung 13 17, 8 29 39, 8 Left Lung 8 10, 9 23 31, 5 Total 21 28, 7 52 71, 3 Tumour mainly at the periphery of the lungs (71. 3%)

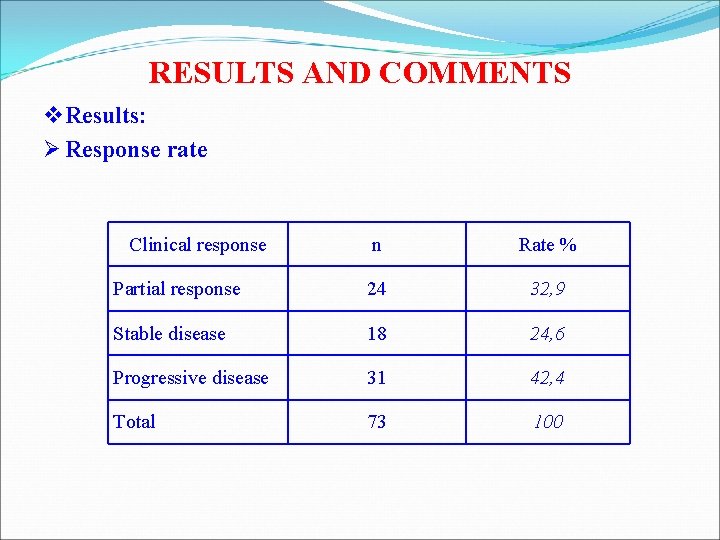
RESULTS AND COMMENTS v Results: Ø Response rate Clinical response n Rate % Partial response 24 32, 9 Stable disease 18 24, 6 Progressive disease 31 42, 4 Total 73 100

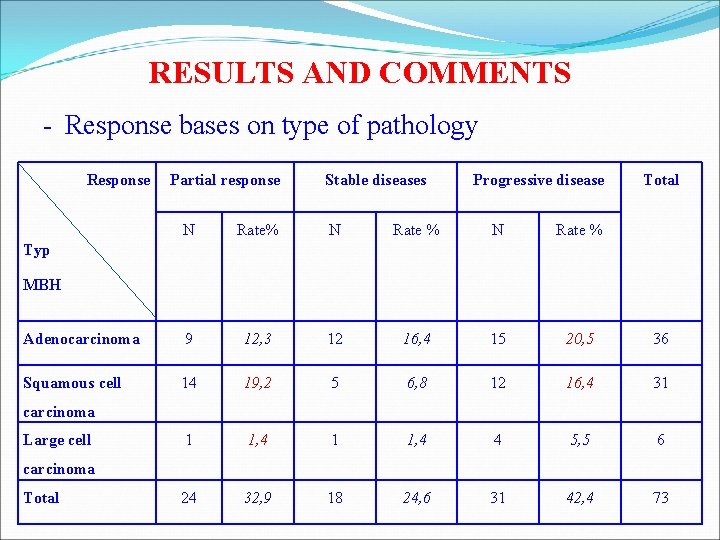
RESULTS AND COMMENTS - Response bases on type of pathology Response Partial response Stable diseases Progressive disease Total N Rate% N Rate % Adenocarcinoma 9 12, 3 12 16, 4 15 20, 5 36 Squamous cell 14 19, 2 5 6, 8 12 16, 4 31 1 1, 4 4 5, 5 6 24 32, 9 18 24, 6 31 42, 4 73 Typ MBH carcinoma Large cell carcinoma Total

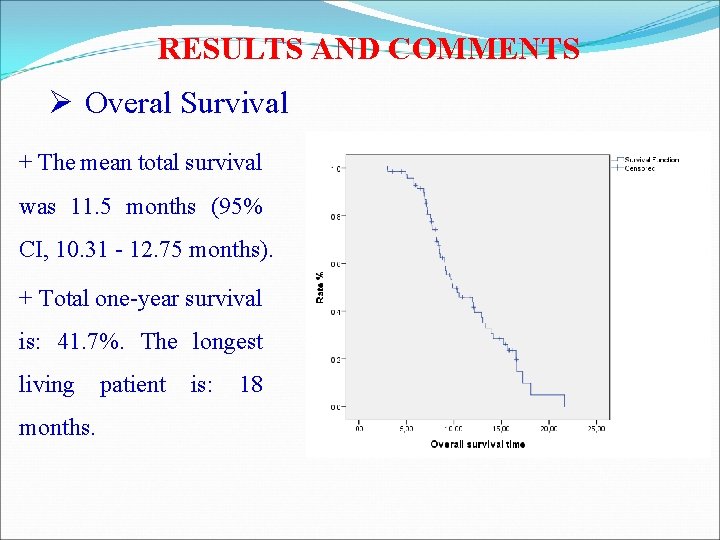
RESULTS AND COMMENTS Ø Overal Survival + The mean total survival was 11. 5 months (95% CI, 10. 31 - 12. 75 months). + Total one-year survival is: 41. 7%. The longest living patient is: 18 months.

RESULTS AND COMMENTS ØOverall survival compare smokers and non-smokers group. The mean survival of the non-smokers group was 12. 74 months, compared with 10. 32 months of the smokers group. However, the difference was not statistically significant (p> 0. 05).

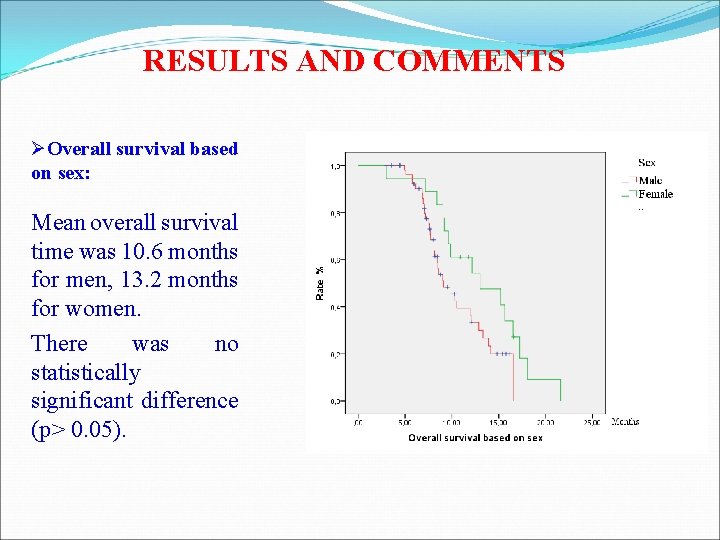
RESULTS AND COMMENTS ØOverall survival based on sex: Mean overall survival time was 10. 6 months for men, 13. 2 months for women. There was no statistically significant difference (p> 0. 05).

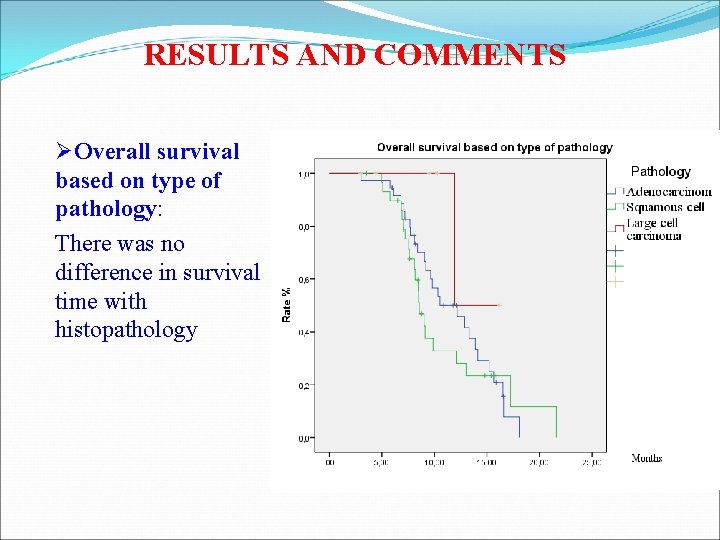
RESULTS AND COMMENTS ØOverall survival based on type of pathology: There was no difference in survival time with histopathology

RESULTS AND COMMENTS Ø Compared to other authors Authors N Overall survival Total response rate (month) % Ying Li (2014) 34 12, 5 29, 4 Yun Fan (2010) 27 13 33, 3 Khurum Khan (2013) 22 10, 4 15 ECOG 1594 260 8, 1 21 Vu van vu (2002) 31 10, 2 31 My study 73 11, 5 32, 9

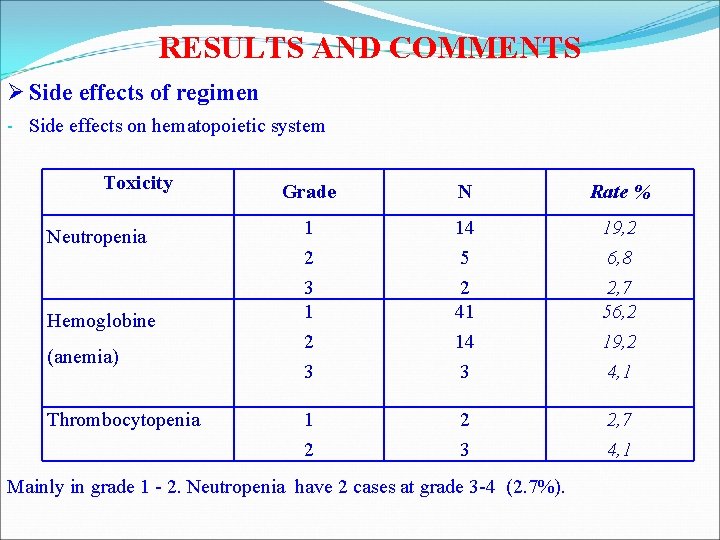
RESULTS AND COMMENTS Ø Side effects of regimen - Side effects on hematopoietic system Toxicity Neutropenia Hemoglobine (anemia) Thrombocytopenia Grade N Rate % 1 2 3 14 5 2 41 14 3 19, 2 6, 8 2, 7 56, 2 19, 2 4, 1 1 2 2 3 2, 7 4, 1 Mainly in grade 1 - 2. Neutropenia have 2 cases at grade 3 -4 (2. 7%).

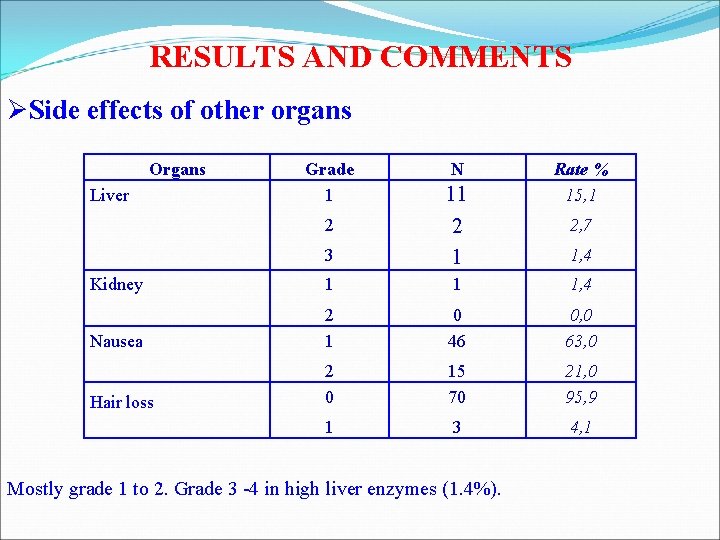
RESULTS AND COMMENTS ØSide effects of other organs Organs Grade 1 N Rate % 15, 1 3 11 2 1 Kidney 1 1 1, 4 Nausea 2 1 0 46 0, 0 63, 0 Hair loss 2 0 15 70 21, 0 95, 9 1 3 4, 1 Liver 2 Mostly grade 1 to 2. Grade 3 -4 in high liver enzymes (1. 4%). 2, 7 1, 4

CONCLUSIONS v Clinical and subclinical characteristics: - NSCLC patients often in > 40 years of age, peak between 50 and 69 years. - Scale: male / female : 3 / 1 - Common clinical symptoms : Cough (69, 9%), chest pain (87, 7%), dysnea (41, 1%) - Pierre Marie Syndrome (10, 9%) Pancoat Tobiat syndrome (22%).

CONCLUSIONS - Tumour situation: Left lung: 42, 4% Right lung: 57, 6% - Tumour mainly at the periphery of the lungs (71. 3%) - Adenocarcinoma: 49, 3%, Squamous cell carcinoma 42, 5%

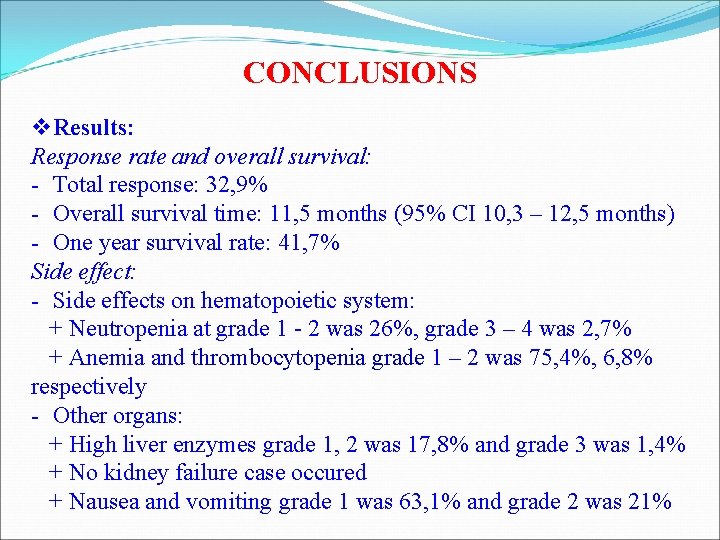
CONCLUSIONS v Results: Response rate and overall survival: - Total response: 32, 9% - Overall survival time: 11, 5 months (95% CI 10, 3 – 12, 5 months) - One year survival rate: 41, 7% Side effect: - Side effects on hematopoietic system: + Neutropenia at grade 1 - 2 was 26%, grade 3 – 4 was 2, 7% + Anemia and thrombocytopenia grade 1 – 2 was 75, 4%, 6, 8% respectively - Other organs: + High liver enzymes grade 1, 2 was 17, 8% and grade 3 was 1, 4% + No kidney failure case occured + Nausea and vomiting grade 1 was 63, 1% and grade 2 was 21%

PROPOSALS Ø - Gemcitabine - Cisplatin is a safe and effective regimen for patients with late-stage NSCLC, and should therefore continue to be widely used in clinical Ø - More studies on this regimen as well as other regimens should be made at the National Lung Hospital to compare the efficacy of the regimens.

THANK YOU
 độc tọa u hoàng lý
độc tọa u hoàng lý Châu đại dương tiếp giáp với đại dương nào
Châu đại dương tiếp giáp với đại dương nào Duang duen nnn
Duang duen nnn Dr duong tuan nguyen
Dr duong tuan nguyen Thanh cánh thượng thanh cánh hạ
Thanh cánh thượng thanh cánh hạ Thanh binh nguyen
Thanh binh nguyen Nhà nguyễn thành lập
Nhà nguyễn thành lập Nguyen tan khoi nguyen
Nguyen tan khoi nguyen Bmc oncology department
Bmc oncology department @quithch1
@quithch1 Ellen duong
Ellen duong An hy quan
An hy quan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Dương văn nội
Dương văn nội Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Phosim
Phosim Khi trong phương đông vừa hé ánh dương
Khi trong phương đông vừa hé ánh dương Ninh duong lan ngoc
Ninh duong lan ngoc Khúc thừa dụ quê ở đâu
Khúc thừa dụ quê ở đâu Các loại dương xỉ thường gặp
Các loại dương xỉ thường gặp Define hospital in pharmacy
Define hospital in pharmacy Hesf1
Hesf1 Hospital department structure
Hospital department structure Flagler hospital billing
Flagler hospital billing Cappagh hospital hip replacement
Cappagh hospital hip replacement National hospital care survey
National hospital care survey 2013 hospital national patient safety goals
2013 hospital national patient safety goals National hospital ambulatory medical care survey
National hospital ambulatory medical care survey National pediatric hospital phnom penh
National pediatric hospital phnom penh National risk and resilience department
National risk and resilience department National core standard
National core standard Function of national audit department in malaysia
Function of national audit department in malaysia Hãy giơ tay lên hướng về nơi thánh
Hãy giơ tay lên hướng về nơi thánh Hoa cà kết thành quả gì
Hoa cà kết thành quả gì