National Academy of Sciences of Ukraine State Scientific



![Scheme of mordenite impregnation with calix[6]arene Mordenite soaked in Na. Cl solution during 24 Scheme of mordenite impregnation with calix[6]arene Mordenite soaked in Na. Cl solution during 24](https://slidetodoc.com/presentation_image/38ecbe86557f0731faf884f42f006155/image-12.jpg)
![IR spectra of mordenite before and after impregnation with calix[6]arene Sr recovery rates in IR spectra of mordenite before and after impregnation with calix[6]arene Sr recovery rates in](https://slidetodoc.com/presentation_image/38ecbe86557f0731faf884f42f006155/image-13.jpg)


- Slides: 15

National Academy of Sciences of Ukraine State Scientific Institution “Institute for Single Crystals” (SSI ISC NASU) A. V. GRYGOROVA, K. YU. BRYLEVA, A. YU. ANDRYUSHCHENKO, N. N. GREBENYUK, I. B. SHCHERBAKOV, K. N. BELIKOV Sr and Cs selective calixarene-based sorbents: analytical application in environmental chemistry

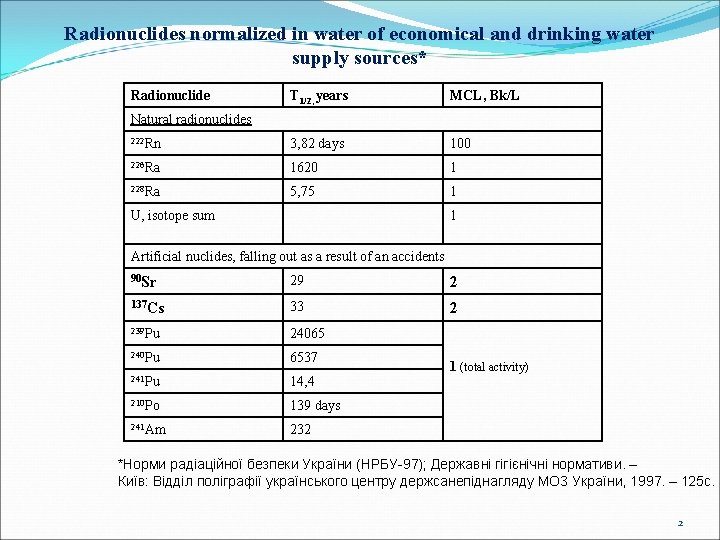
Radionuclides normalized in water of economical and drinking water supply sources* Radionuclide Т 1/2, years MCL, Bk/L 222 Rn 3, 82 days 100 226 Ra 1620 1 228 Ra 5, 75 1 Natural radionuclides U, isotope sum 1 Artificial nuclides, falling out as a result of an accidents 90 Sr 29 2 137 Cs 33 2 239 Pu 24065 240 Pu 6537 241 Pu 14, 4 210 Po 139 days 241 Am 232 1 (total activity) *Норми радіаційної безпеки України (НРБУ-97); Державні гігієнічні нормативи. – Київ: Відділ поліграфії українського центру держсанепіднагляду МОЗ України, 1997. – 125 с. 2

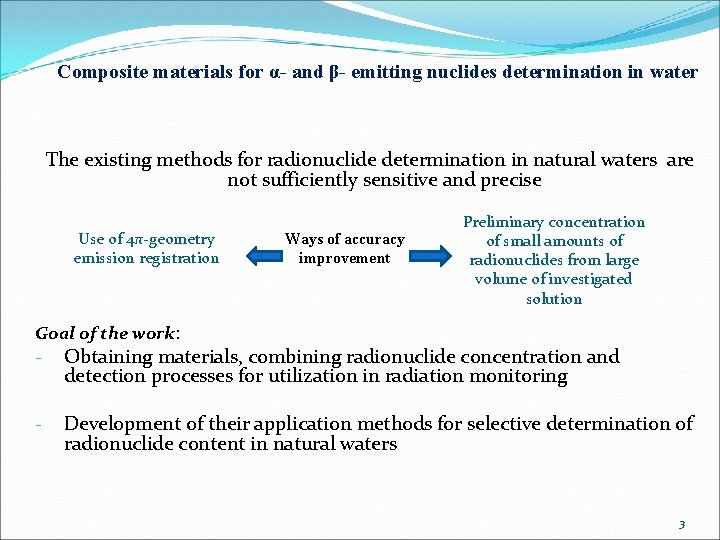
Composite materials for α- and β- emitting nuclides determination in water The existing methods for radionuclide determination in natural waters are not sufficiently sensitive and precise Use of 4π-geometry emission registration Ways of accuracy improvement Preliminary concentration of small amounts of radionuclides from large volume of investigated solution Goal of the work: - Obtaining materials, combining radionuclide concentration and detection processes for utilization in radiation monitoring - Development of their application methods for selective determination of radionuclide content in natural waters 3

Methods Drawbacks Flow detectors in combination Short counting time; poor resolution; detection limits for αwith extraction or chromatography emitters -500 Bk/L, for β-emitters – 350 Bk/L Liquid scintillating detectors in combination with extraction or chromatography Deterioration of scintillation characteristics when large volume of water is used; high detection limits. Porous scintillators with branchy surface 2 -π registration geometry; clogging up of surface with radionuclides. Radiochemical methods 21 stage of a sample preparation; activity measurement directly after separation stage and in 2 -4 weeks, that is after equilibrium between Sr-90 and Y-90 is achieved. 4

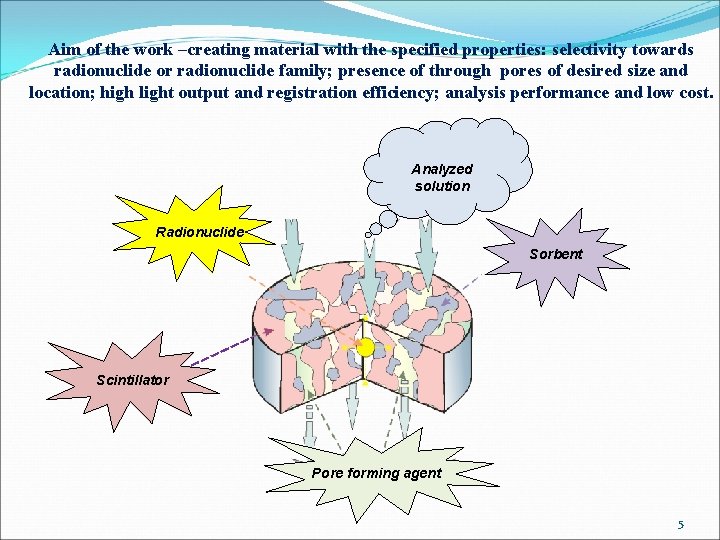
Aim of the work –creating material with the specified properties: selectivity towards radionuclide or radionuclide family; presence of through pores of desired size and location; high light output and registration efficiency; analysis performance and low cost. Analyzed solution Radionuclide Sorbent Scintillator Pore forming agent 5

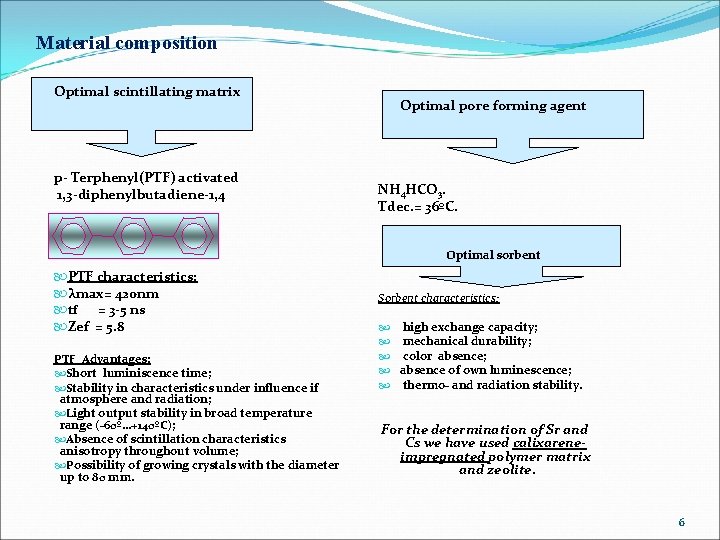
Material composition Optimal scintillating matrix p- Terphenyl(PTF) activated 1, 3 -diphenylbutadiene-1, 4 Optimal pore forming agent NH 4 HCO 3. Tdec. = 36ºC. Optimal sorbent PTF characteristics: λmax= 420 nm tf = 3 -5 ns Zef = 5. 8 PTF Advantages: Short luminiscence time; Stability in characteristics under influence if atmosphere and radiation; Light output stability in broad temperature range (-60º…+140ºС); Absence of scintillation characteristics anisotropy throughout volume; Possibility of growing crystals with the diameter up to 80 mm. Sorbent characteristics: high exchange capacity; mechanical durability; color absence; absence of own luminescence; thermo- and radiation stability. For the determination of Sr and Cs we have used calixareneimpregnated polymer matrix and zeolite. 6

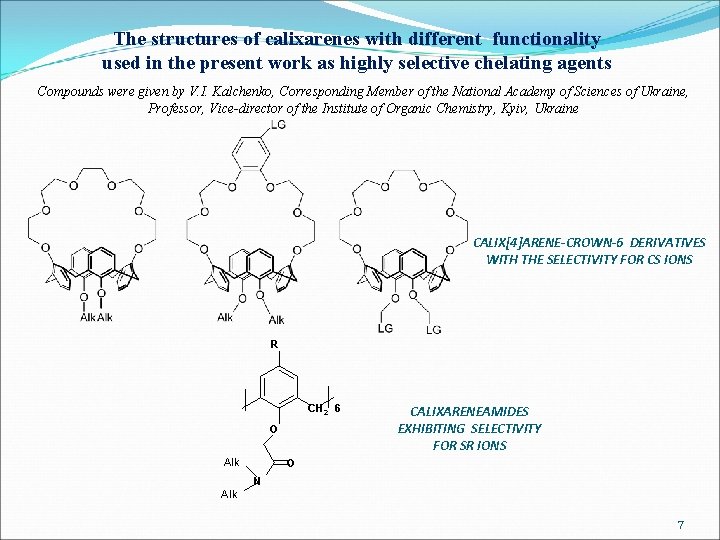
The structures of calixarenes with different functionality used in the present work as highly selective chelating agents Compounds were given by V. I. Kalchenko, Corresponding Member of the National Academy of Sciences of Ukraine, Professor, Vice-director of the Institute of Organic Chemistry, Kyiv, Ukraine CALIX[4]ARENE-CROWN-6 DERIVATIVES WITH THE SELECTIVITY FOR CS IONS R CH 2 6 O A lk CALIXARENEAMIDES EXHIBITING SELECTIVITY FOR SR IONS O N A lk 7

SEM images of polymer spheres cross-section in back-scattered electron mode 8

Investigation methods Inductively coupled plasma atomic emission spectrometry (ICP-AES), Thermo Jarrell Ash, USA Operating conditions λ (Sr) RF Power Plasma gas flow Auxiliary gas flow Nebulizer gas flow Pump rate Solution uptake rate Integration time 421. 55 /407. 77 nm 1150 Wt 14 L/min 1 L/min 36 psi (2, 5 atm) 100 rpm 1, 85 m. L/min 2 s Flame atomic emission spectrometry Saturn, USSR Operating conditions λ (Cs) Flame Integration time Slit width Grating density 852. 1 nm Acetylene-Air 1 s 0. 2 nm 1200 nm-1 9

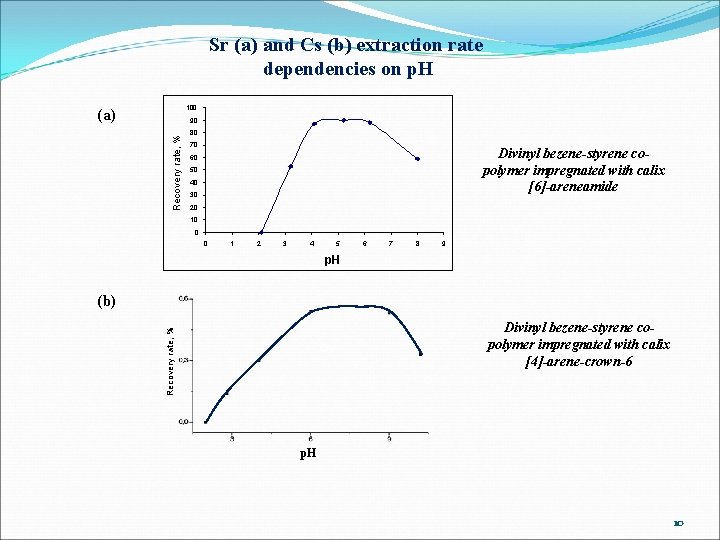
Sr (a) and Cs (b) extraction rate dependencies on p. H 100 (a) Recovery rate, % 90 80 70 Divinyl bezene-styrene copolymer impregnated with calix [6]-areneamide 60 50 40 30 20 10 0 0 1 2 3 4 5 6 7 8 9 p. H (b) Recovery rate, % Divinyl bezene-styrene copolymer impregnated with calix [4]-arene-crown-6 p. H 10

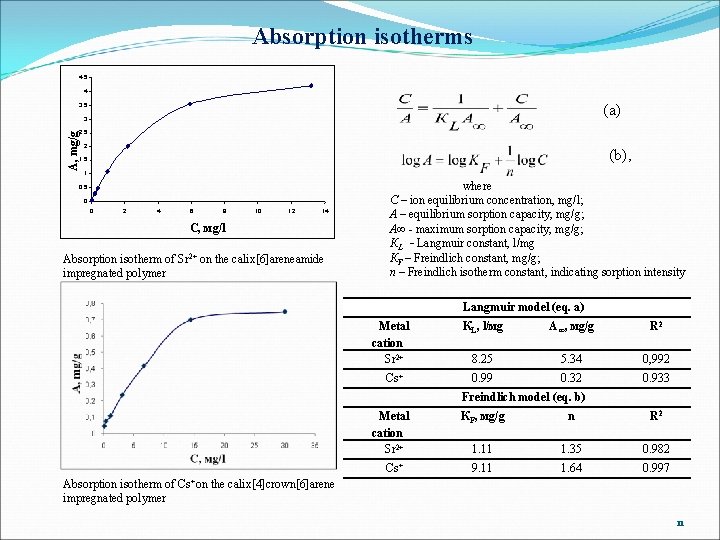
Absorption isotherms 4, 5 4 (a) 3, 5 3 А, mg/g 2, 5 2 (b), 1, 5 1 0, 5 0 0 2 4 6 8 10 12 14 C, мg/l Absorption isotherm of Sr 2+ on the calix[6]areneamide impregnated polymer where C – ion equilibrium concentration, mg/l; A – equilibrium sorption capacity, mg/g; A∞ - maximum sorption capacity, mg/g; КL - Langmuir constant, l/mg КF – Freindlich constant, mg/g; n – Freindlich isotherm constant, indicating sorption intensity Langmuir model (eq. a) Metal cation Sr 2+ Cs+ КL, l/мg А ∞, мg/g R 2 8. 25 5. 34 0, 992 0. 99 0. 32 0. 933 Freindlich model (eq. b) Metal cation Sr 2+ Cs+ КF, мg/g n R 2 1. 11 1. 35 0. 982 9. 11 1. 64 0. 997 Absorption isotherm of Cs+on the calix[4]crown[6]arene impregnated polymer 11
![Scheme of mordenite impregnation with calix6arene Mordenite soaked in Na Cl solution during 24 Scheme of mordenite impregnation with calix[6]arene Mordenite soaked in Na. Cl solution during 24](https://slidetodoc.com/presentation_image/38ecbe86557f0731faf884f42f006155/image-12.jpg)
Scheme of mordenite impregnation with calix[6]arene Mordenite soaked in Na. Cl solution during 24 hours at ambient temperature Mordenite in -Na form d < 0. 125 mm Washing with distilled water Washing with distilled Washing with methanol water Treated actively during 60 min Drying at 50ºC during 3 hours 1 g of calix[6]arene dissolved in 10 ml of CH 2 Cl 2 Impregnation tri-n-butyl phosphate dissolved in CH 2 Cl 2 Removing diluent at 100ºC Drying at 120ºC 12
![IR spectra of mordenite before and after impregnation with calix6arene Sr recovery rates in IR spectra of mordenite before and after impregnation with calix[6]arene Sr recovery rates in](https://slidetodoc.com/presentation_image/38ecbe86557f0731faf884f42f006155/image-13.jpg)
IR spectra of mordenite before and after impregnation with calix[6]arene Sr recovery rates in systems containing calixarene-impregnated mordenite Distilled water Model solution Without calixarene, model solution 40% 4% 8% 13

Prospective work • Design of highly selective scintillating sorbent with the use of materials investigated • Investigation and optimization of physicochemical parameters of the composite materials obtained • Developing the experimental technique for measuring the radionuclide concentration in water samples with the use of porous scintillator obtained The work is supported by STCU Grant No 4955 “Composite materials on the basis of highly selective calixarene sorbents for determination of radionuclides in environments” 14

Thank you very much for your kind attention! 15
 Railroad training school
Railroad training school National academy of sciences forensic science
National academy of sciences forensic science Human science tok
Human science tok Ukraine national symbols
Ukraine national symbols Accreditation ukraine
Accreditation ukraine National accreditation agency of ukraine
National accreditation agency of ukraine Bila tserkva university
Bila tserkva university Ukrainian national library
Ukrainian national library State service of ukraine on personal data protection
State service of ukraine on personal data protection State nuclear regulatory inspectorate
State nuclear regulatory inspectorate Hazleton area academy of sciences
Hazleton area academy of sciences Academy of motion picture arts and sciences benefits
Academy of motion picture arts and sciences benefits Hawaii academy of arts and science
Hawaii academy of arts and science Putnam academy of arts and science
Putnam academy of arts and science Nuclear safety institute of the russian academy of sciences
Nuclear safety institute of the russian academy of sciences Uzbek academy of sciences
Uzbek academy of sciences