National 5 Chemistry Unit 2 Natures Chemistry Section










- Slides: 46

National 5 Chemistry Unit 2 – Nature’s Chemistry Section 7 – Everyday Consumer Products

Section 7 – Alcohols Pupils should be able to… • Identify that an alcohol molecule contains –OH as its functional group • State that alcohols are straight chain molecules and can be represented by a general formula • State that alcohols are used a fuels as they are highly flammable. • State that they burn with clean flames • State that alcohols are often used as solvents • State that methanol, ethanol and propanol are miscible in water • State that after propanol solubility decreases as the size of the alcohol molecule increases • State and explain why as alcohol molecular size increases, the strength of the intermolecular force increases • Name straight chain alcohols containing no more that 8 carbons • Write molecular formulae and draw structural formulae from systematic names

What is a Homologous Series? Homologous Series – A group of chemically similar compounds which can be represented by a general formula. – Physical properties change gradually as you go down the series There are two groups that we are going to look at 1. Alcohol 2. Carboxylic acids


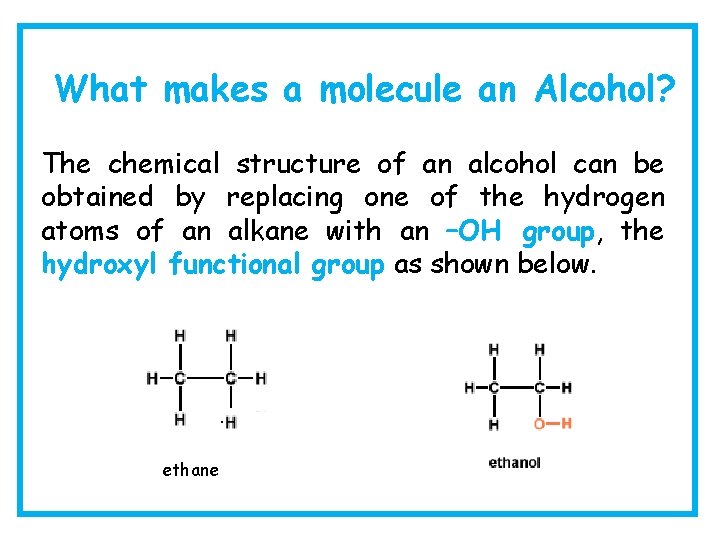
What makes a molecule an Alcohol? The chemical structure of an alcohol can be obtained by replacing one of the hydrogen atoms of an alkane with an –OH group, the hydroxyl functional group as shown below. ethane

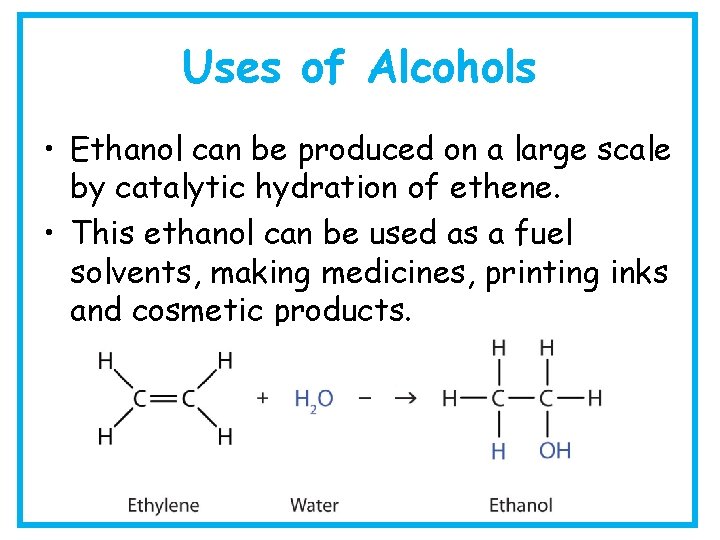
Uses of Alcohols • Ethanol can be produced on a large scale by catalytic hydration of ethene. • This ethanol can be used as a fuel solvents, making medicines, printing inks and cosmetic products.

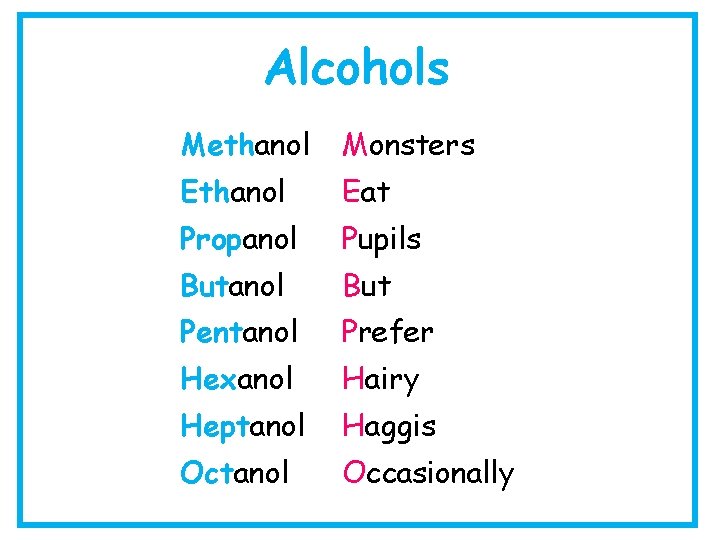
Alcohols Methanol Monsters Ethanol Eat Propanol Pupils Butanol But Pentanol Prefer Hexanol Hairy Heptanol Haggis Octanol Occasionally

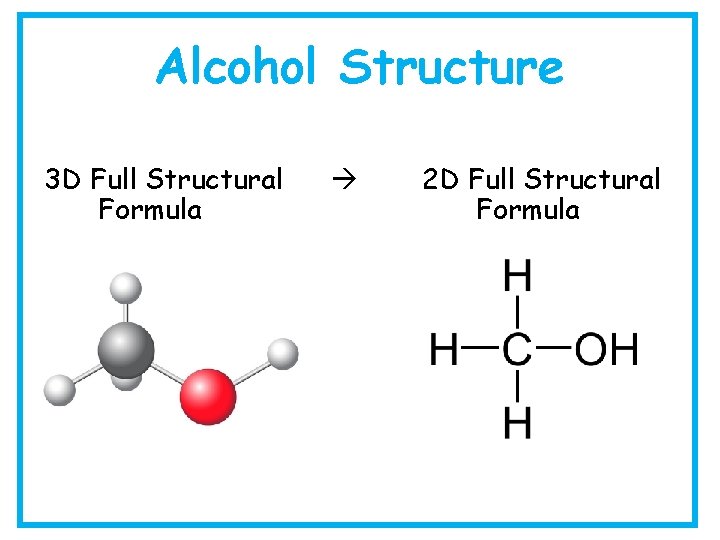
Alcohol Structure 3 D Full Structural Formula 2 D Full Structural Formula

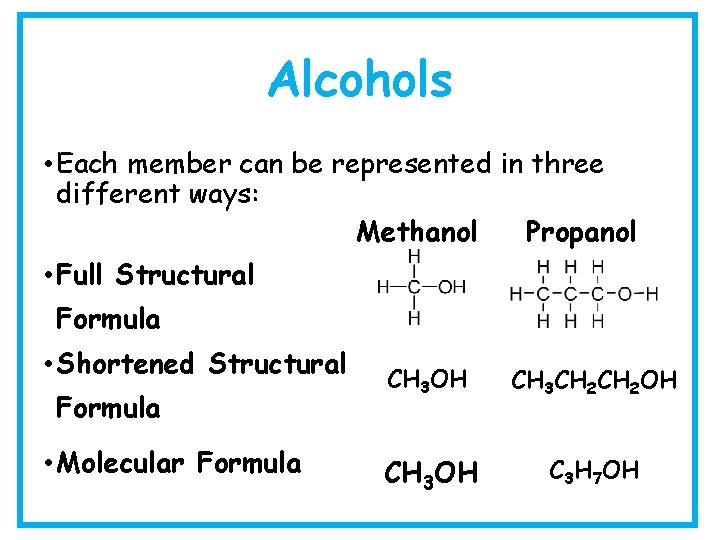
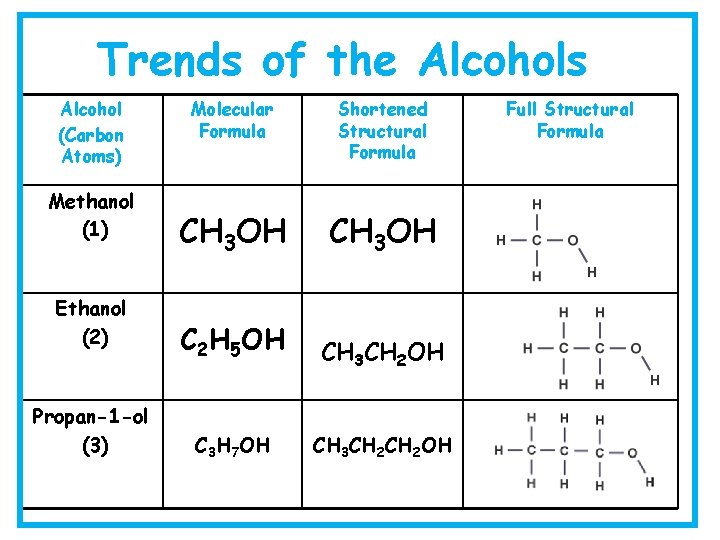
Alcohols • Each member can be represented in three different ways: Methanol Propanol • Full Structural Formula • Shortened Structural Formula • Molecular Formula CH 3 OH CH 3 CH 2 OH CH 3 OH C 3 H 7 OH

Alcohol Information • The alcohols are a family of hydrocarbons that all share similar properties. • Single Carbon to carbon bonds (- C – C -) • Burn in plentiful of oxygen to produce carbon dioxide and water • Saturated hydrocarbons • Highly flammable • All share a General Formula

Trends of the Alcohols Alcohol (Carbon Atoms) Methanol (1) Ethanol (2) Propan-1 -ol (3) Molecular Formula Shortened Structural Formula CH 3 OH C 2 H 5 OH CH 3 CH 2 OH C 3 H 7 OH CH 3 CH 2 OH Full Structural Formula

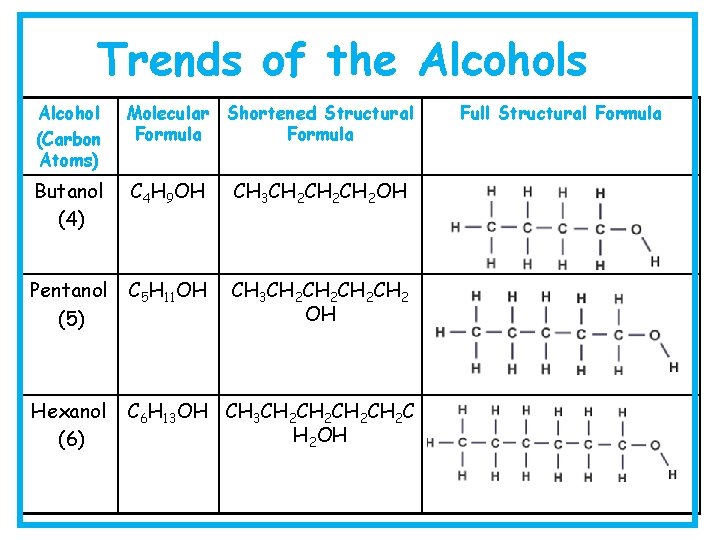
Trends of the Alcohols Alcohol (Carbon Atoms) Molecular Shortened Structural Formula Butanol (4) C 4 H 9 OH CH 3 CH 2 CH 2 OH Pentanol (5) C 5 H 11 OH CH 3 CH 2 CH 2 OH Hexanol (6) C 6 H 13 OH CH 3 CH 2 CH 2 C H 2 OH Full Structural Formula

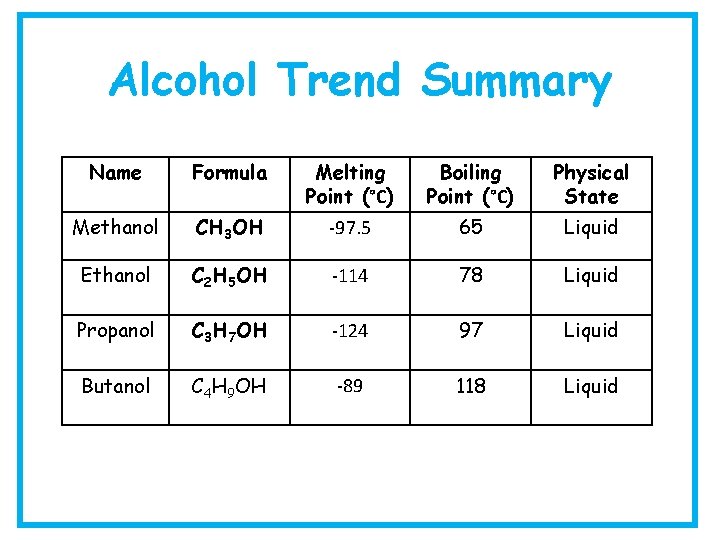
Alcohol Trend Summary Name Formula Melting Point (°C) Boiling Point (°C) Physical State Methanol CH 3 OH -97. 5 65 Liquid Ethanol C 2 H 5 OH -114 78 Liquid Propanol C 3 H 7 OH -124 97 Liquid Butanol C 4 H 9 OH -89 118 Liquid

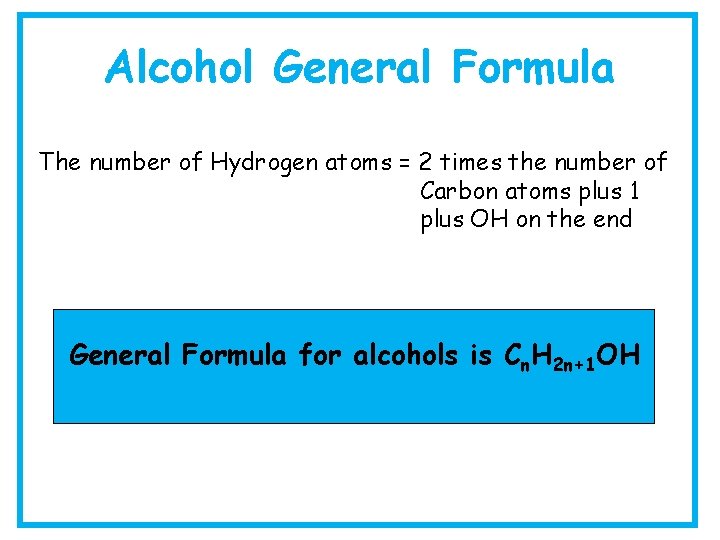
Alcohol General Formula The number of Hydrogen atoms = 2 times the number of Carbon atoms plus 1 plus OH on the end General Formula for alcohols is Cn. H 2 n+1 OH

Alcohols – Physical Properties • B. p – gradual increase from methanol to octanol • Viscosity - increases as molecular size increases • Flammability – burns with a clean flame • Miscibility - methanol, ethanol and propanol are miscible in water • Solubility –solubility decreases after propanol as the size of the alcohol molecule increases

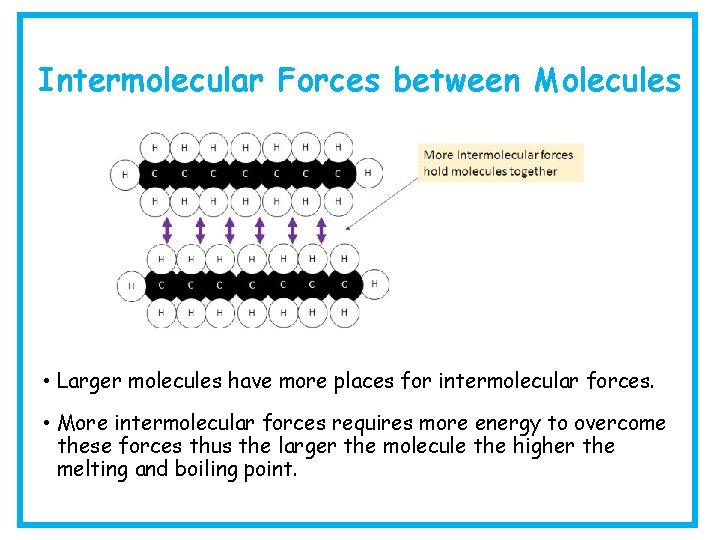
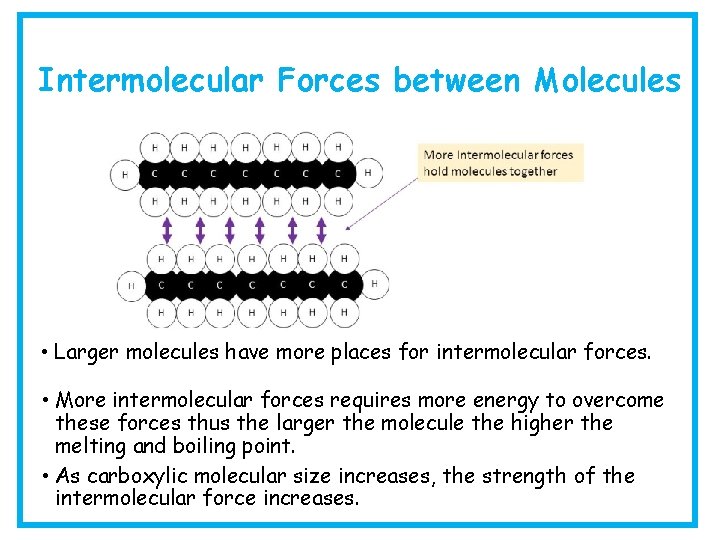
Intermolecular Forces between Molecules • Larger molecules have more places for intermolecular forces. • More intermolecular forces requires more energy to overcome these forces thus the larger the molecule the higher the melting and boiling point.

Naming Alcohols 1. Find the longest chain which includes the functional group. This will form the last part of the name. 2. Number the main chain so that it gives the functional lower number over positions to the side groups. 3. Side branch names end in “yl” and depend on the number of carbon atoms in them; 1 = methyl 2 = ethyl 3 = propyl etc 4. Alphabetically order is used if different side branches appear in the same structure e. g. ethyl before methyl 5. Hyphens are used before or after numbers that come next to letters within a name e. g. (2 -ethyl-3 -methyl…. . ) 6. Commas are used between numbers if there is more than one of the same side branch (e. g. 2, 3, 3 – trimethyl…. )

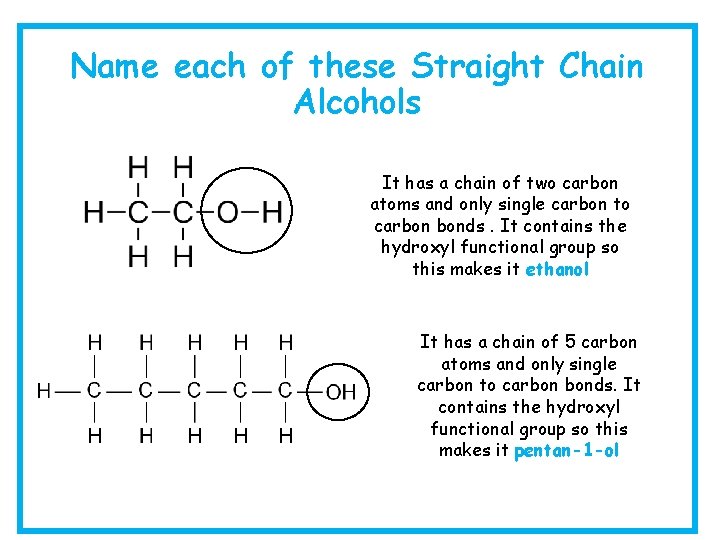
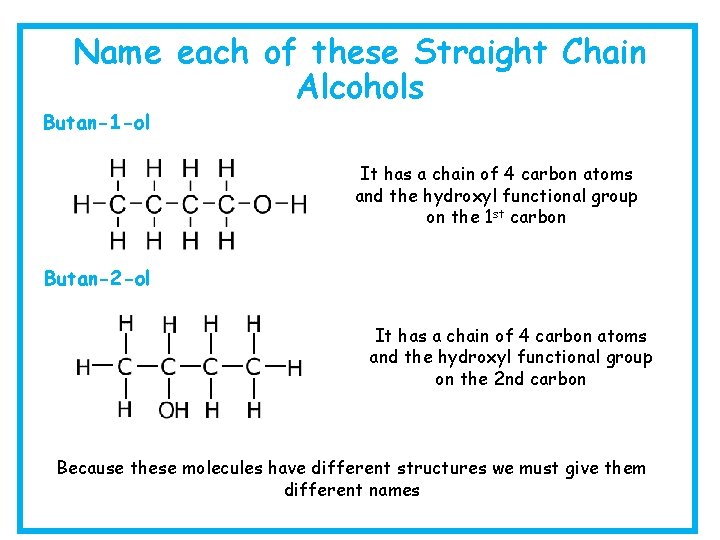
Name each of these Straight Chain Alcohols It has a chain of two carbon atoms and only single carbon to carbon bonds. It contains the hydroxyl functional group so this makes it ethanol It has a chain of 5 carbon atoms and only single carbon to carbon bonds. It contains the hydroxyl functional group so this makes it pentan-1 -ol

Name each of these Straight Chain Alcohols Butan-1 -ol It has a chain of 4 carbon atoms and the hydroxyl functional group on the 1 st carbon Butan-2 -ol It has a chain of 4 carbon atoms and the hydroxyl functional group on the 2 nd carbon Because these molecules have different structures we must give them different names

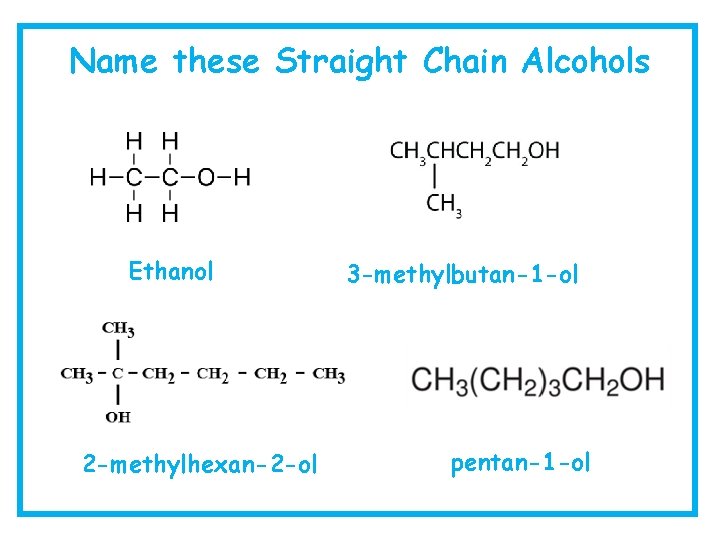
Name these Straight Chain Alcohols Ethanol 2 -methylhexan-2 -ol 3 -methylbutan-1 -ol pentan-1 -ol

Section 7 – Carboxylic Acids Pupils should be able to… • Identify that a carboxylic acid molecule contains –COOH as its functional group • State that carboxylic acids are straight chain molecules and can be represented by a general formula • State uses of carboxylic acids as soaps, medicines and in the preparation of preservatives • Discuss the chemical and physical properties of vinegar a solution of ethanoic acid • State that methanoic, propanoic and butanoic are miscible in water • State that after butanoic solubility decreases as the size of the acid molecule increases • State and explain why as carboxylic acid molecular size increases, the strength of the intermolecular force increases • Name straight chain carboxylic acids containing no more that 8 carbons

Section 7 – Carboxylic Acids Pupils should be able to… • Write molecular formulae and draw structural formulae from systematic names • State that solutions of carboxylic acids have a p. H less than 7. • State that they can act as a base to react with metals, metal oxides, hydroxides and carbonates to form salts • Name salts formed from straight chain carboxylic acids containing no more that 8 carbons

Carboxylic Acids • Carboxylic acids are a group of important straight chain organic chemicals. Vinegar contains ethanoic acid, which is a carboxylic acid. All carboxylic acids have a –COOH functional group, and have similar reactions as a result. • The carboxylic acids are a homologous series of organic compounds so they can be represented by a general formula. • The names of carboxylic acids end in ‘-oic acid’ – eg ethanoic acid.

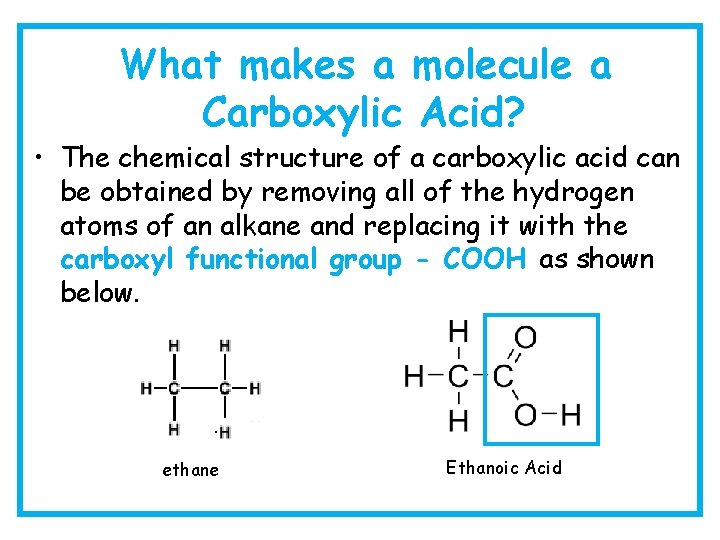
What makes a molecule a Carboxylic Acid? • The chemical structure of a carboxylic acid can be obtained by removing all of the hydrogen atoms of an alkane and replacing it with the carboxyl functional group - COOH as shown below. ethane Ethanoic Acid

Carboxylic Acids Methanoic Monsters Ethanoic Eat Propanoic Pupils Butanoic But Pentanoic Prefer Hexanoic Hairy Heptanoic Haggis Octanoic Occasionally

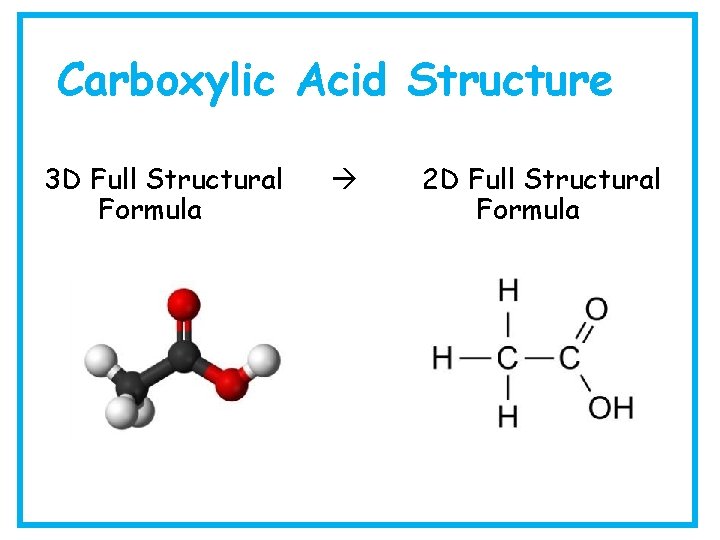
Carboxylic Acid Structure 3 D Full Structural Formula 2 D Full Structural Formula

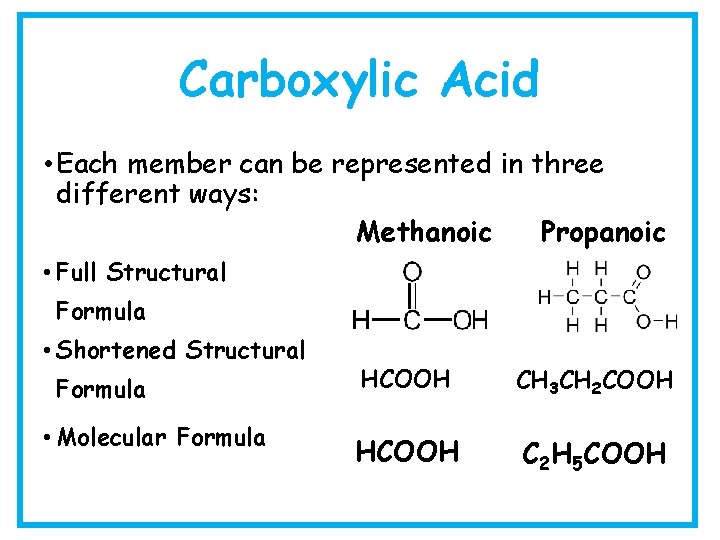
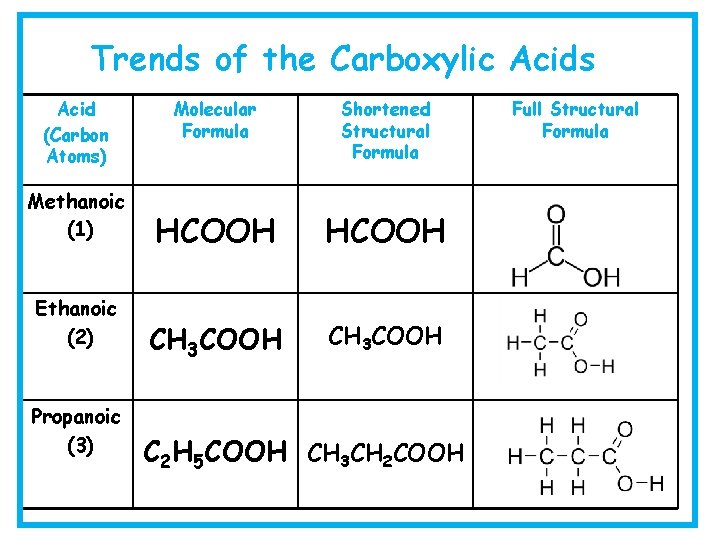
Carboxylic Acid • Each member can be represented in three different ways: Methanoic Propanoic • Full Structural Formula • Shortened Structural Formula • Molecular Formula HCOOH CH 3 CH 2 COOH HCOOH C 2 H 5 COOH

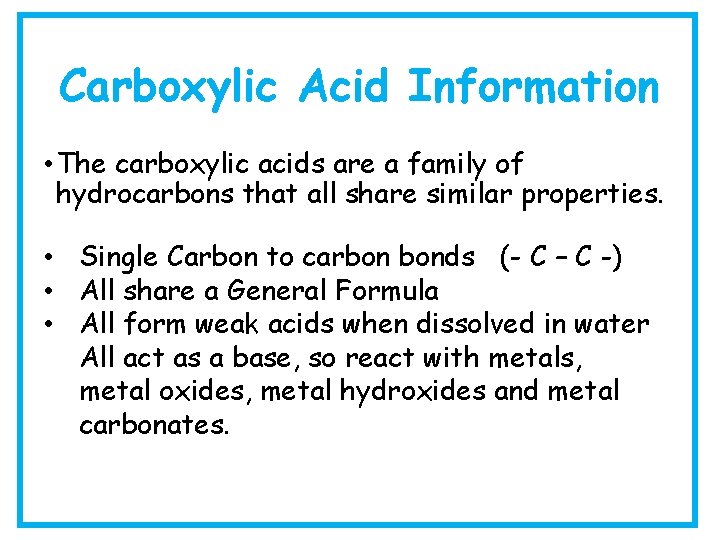
Carboxylic Acid Information • The carboxylic acids are a family of hydrocarbons that all share similar properties. • Single Carbon to carbon bonds (- C – C -) • All share a General Formula • All form weak acids when dissolved in water All act as a base, so react with metals, metal oxides, metal hydroxides and metal carbonates.

Trends of the Carboxylic Acids Acid (Carbon Atoms) Methanoic (1) Ethanoic (2) Propanoic (3) Molecular Formula Shortened Structural Formula HCOOH CH 3 COOH C 2 H 5 COOH CH 3 CH 2 COOH Full Structural Formula

Trends of the Carboxylic Acids Acid (Carbon Atoms) Butanoic (4) Pentanoic (5) Hexanoic (6) Molecular Formula Shortened Structural Formula C 3 H 7 COOH CH 3 CH 2 COOH C 4 H 9 COOH CH 3 CH 2 CH 2 COOH C 5 H 11 COOH CH 3 CH 2 CH 2 COOH Full Structural Formula

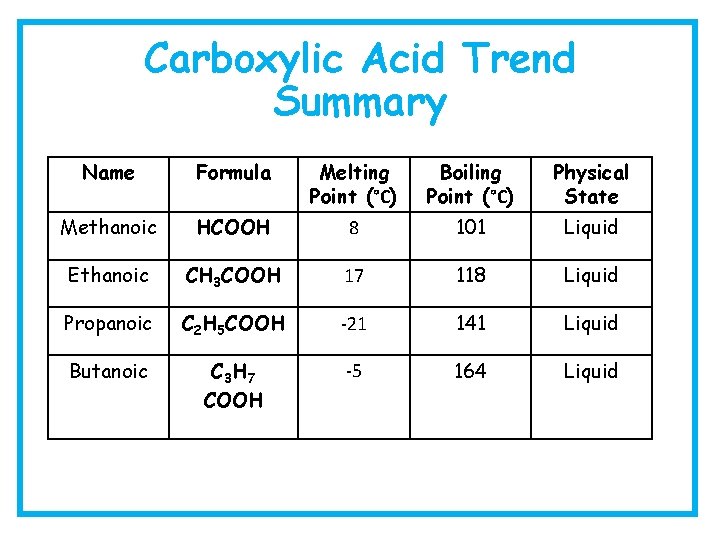
Carboxylic Acid Trend Summary Name Formula Melting Point (°C) Boiling Point (°C) Physical State Methanoic HCOOH 8 101 Liquid Ethanoic CH 3 COOH 17 118 Liquid Propanoic C 2 H 5 COOH -21 141 Liquid Butanoic C 3 H 7 COOH -5 164 Liquid

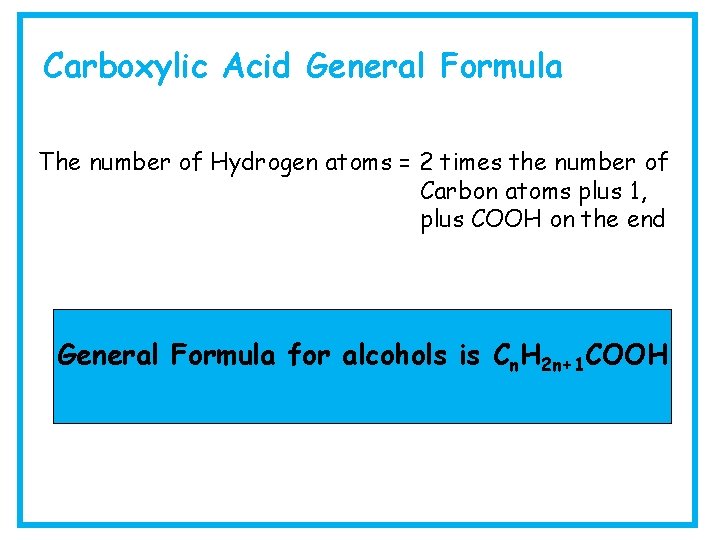
Carboxylic Acid General Formula The number of Hydrogen atoms = 2 times the number of Carbon atoms plus 1, plus COOH on the end General Formula for alcohols is Cn. H 2 n+1 COOH

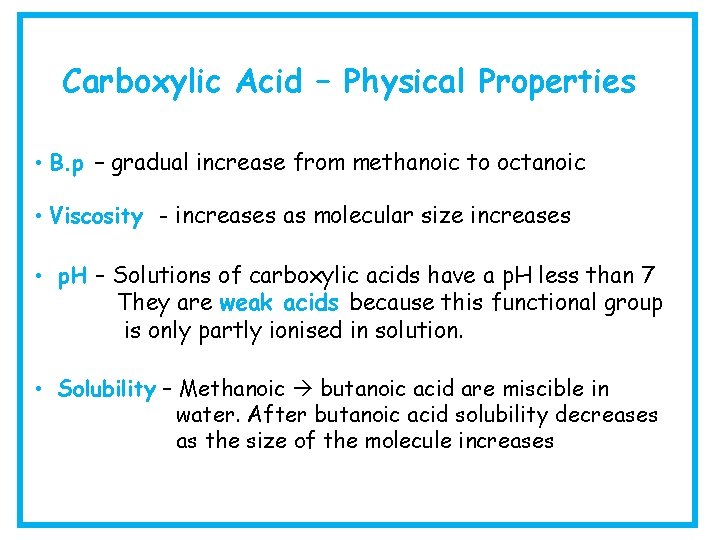
Carboxylic Acid – Physical Properties • B. p – gradual increase from methanoic to octanoic • Viscosity - increases as molecular size increases • p. H – Solutions of carboxylic acids have a p. H less than 7 They are weak acids because this functional group is only partly ionised in solution. • Solubility – Methanoic butanoic acid are miscible in water. After butanoic acid solubility decreases as the size of the molecule increases

Properties of Carboxylic Acids • They dissolve in water to produce acidic solutions (p. H less than 7). • Carboxylic acids can act as a based to react with metals, metal oxides, metal hydroxides and Metal carbonates. • They react with carbonates to produce carbon dioxide, a salt and water. For example: calcium carbonate + ethanoic acid → calcium ethanoate + water + carbon dioxide

Intermolecular Forces between Molecules • Larger molecules have more places for intermolecular forces. • More intermolecular forces requires more energy to overcome these forces thus the larger the molecule the higher the melting and boiling point. • As carboxylic molecular size increases, the strength of the intermolecular force increases.

Naming Carboxylic Acids 1. Find the longest chain which includes the functional group. This will form the last part of the name. 2. Number the main chain so that it gives the functional lower number over positions to the side groups. 3. Side branch names end in “yl” and depend on the number of carbon atoms in them; 1 = methyl 2 = ethyl 3 = propyl etc 4. Alphabetically order is used if different side branches appear in the same structure e. g. ethyl before methyl 5. Hyphens are used before or after numbers that come next to letters within a name e. g. (2 -ethyl-3 -methyl…. . ) 6. Commas are used between numbers if there is more than one of the same side branch (e. g. 2, 3, 3 – trimethyl…. )

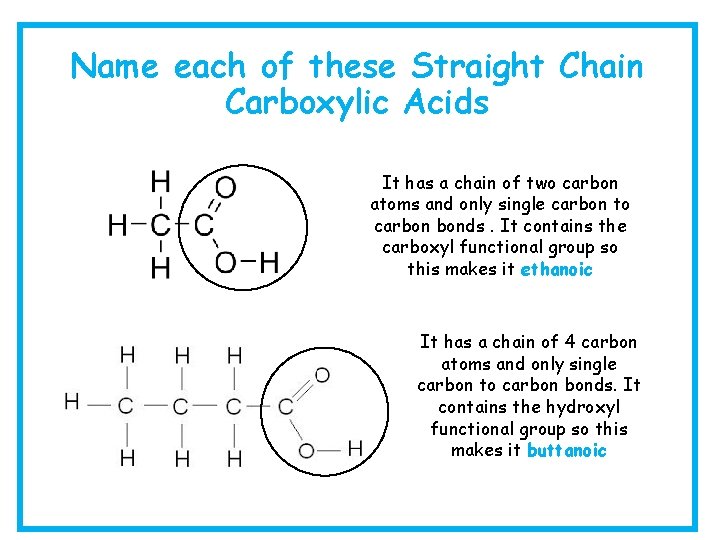
Name each of these Straight Chain Carboxylic Acids It has a chain of two carbon atoms and only single carbon to carbon bonds. It contains the carboxyl functional group so this makes it ethanoic It has a chain of 4 carbon atoms and only single carbon to carbon bonds. It contains the hydroxyl functional group so this makes it buttanoic

Uses of Carboxylic Acids 1. Soaps 2. Medicines 3. Food Preservation 4. Carboxylic Acids in Food

1. Soaps As you should know from Unit 1, acids must produce H+ ions when dissolved in water. Carboxylic acids produce some H+ ions when dissolved in water e. g. CH 3 COOH (aq) CH 3 COO-(aq) + H+(aq) These are known as weak acids as they only partially ionise when they are added to water. HCl and H 2 SO 4 are strong acids as the completely ionise when dissolved in water.

3. Food Preservation Vinegar is a solution of ethanoic acid and is widely used to preserve food. Its low p. H prevents bacteria and fungi from growing. This is an example of pickling e. g. onions and eggs

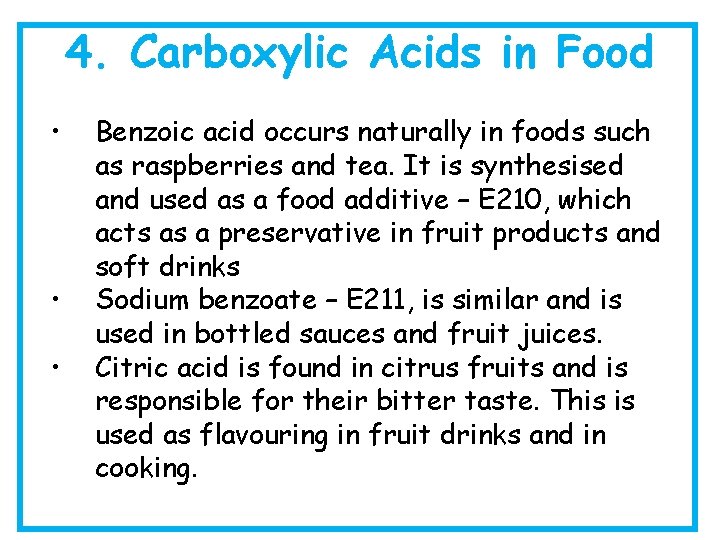
4. Carboxylic Acids in Food • • • Benzoic acid occurs naturally in foods such as raspberries and tea. It is synthesised and used as a food additive – E 210, which acts as a preservative in fruit products and soft drinks Sodium benzoate – E 211, is similar and is used in bottled sauces and fruit juices. Citric acid is found in citrus fruits and is responsible for their bitter taste. This is used as flavouring in fruit drinks and in cooking.

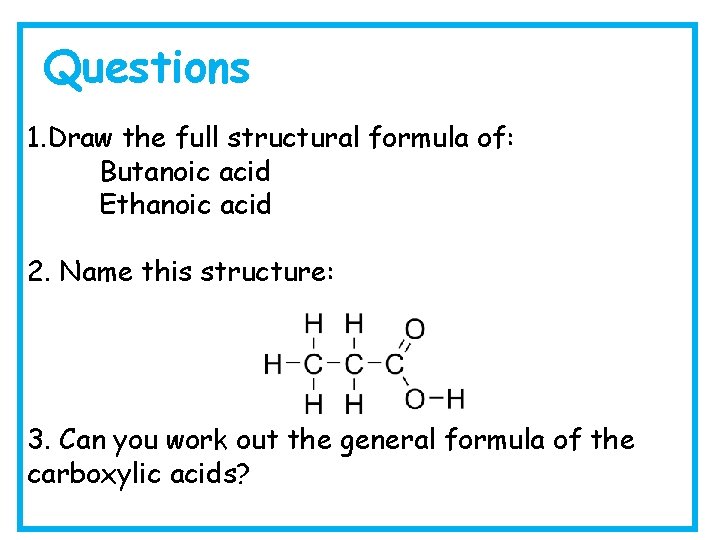
Questions 1. Draw the full structural formula of: Butanoic acid Ethanoic acid 2. Name this structure: 3. Can you work out the general formula of the carboxylic acids?

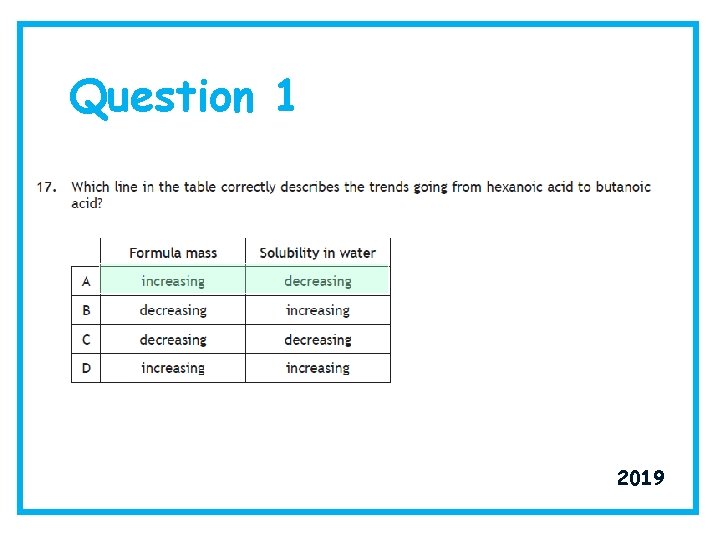
Question 1 2019



Question 2 ?
 Nothing gold can stay subject
Nothing gold can stay subject Natures%20truth
Natures%20truth Describe the nature and scope of psychology
Describe the nature and scope of psychology Natures first green is gold
Natures first green is gold Natures pure purifier
Natures pure purifier Unit 10, unit 10 review tests, unit 10 general test
Unit 10, unit 10 review tests, unit 10 general test National unification and the national state
National unification and the national state National 3 chemistry
National 3 chemistry Ib chemistry functional groups
Ib chemistry functional groups Inorganic vs organic chemistry
Inorganic vs organic chemistry Chapter 10 section 1 the national legislature
Chapter 10 section 1 the national legislature A new national identity section 1
A new national identity section 1 Three fold division of navigable waters
Three fold division of navigable waters Chapter 7 section 4 states rights and the national bank
Chapter 7 section 4 states rights and the national bank A new national identity section 1
A new national identity section 1 Chapter 22 review organic chemistry section 1 answers
Chapter 22 review organic chemistry section 1 answers Chemistry
Chemistry Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chemistry in biology section 2 chemical reactions
Chemistry in biology section 2 chemical reactions Chapter 2 the chemistry of life section review 2-2
Chapter 2 the chemistry of life section review 2-2 Carboxylic acid formula
Carboxylic acid formula Introduction to chemistry section 3 scientific methods
Introduction to chemistry section 3 scientific methods Chemistry in biology chapter 6 section 1 answer key
Chemistry in biology chapter 6 section 1 answer key Chapter 6 section 3 water and solutions
Chapter 6 section 3 water and solutions National risk ambulance
National risk ambulance Unit 5 food national geographic
Unit 5 food national geographic Unit 9 lesson 4
Unit 9 lesson 4 Ap chemistry thermochemistry frq
Ap chemistry thermochemistry frq Chemistry unit 7 molarity
Chemistry unit 7 molarity Q=mc∆t
Q=mc∆t Chemistry semester 2 review unit 12 thermochemistry
Chemistry semester 2 review unit 12 thermochemistry Molal units
Molal units Wjec chemistry unit 2
Wjec chemistry unit 2 Lewis structures cannot
Lewis structures cannot Types of reactions grade 11
Types of reactions grade 11 Chemistry unit 2 study guide answer key
Chemistry unit 2 study guide answer key Chemistry unit review answer key
Chemistry unit review answer key Chemistry grade 10 unit 4
Chemistry grade 10 unit 4 Unit 6 exam chemistry
Unit 6 exam chemistry Ap chemistry molecular geometry
Ap chemistry molecular geometry Higher chemistry unit 3 notes
Higher chemistry unit 3 notes Wjec chemistry unit 2
Wjec chemistry unit 2 Chemistry unit 6 sticky tape post lab
Chemistry unit 6 sticky tape post lab Living by chemistry unit 2 smells answers
Living by chemistry unit 2 smells answers Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Ap chemistry unit 3
Ap chemistry unit 3 Ap chemistry unit 2
Ap chemistry unit 2