NarcoticOpioid analgesics Dr Manoj Kumar Mahapatra Analgesics Relieves













- Slides: 19

Narcotic/Opioid analgesics Dr Manoj Kumar Mahapatra

Analgesics: Relieves pain without affecting its cause. 2 types: Opioid/narcotic analgesics Non-opioid/Non-narcotic analgesics (NSAID) Narcotic analgesics Non-narcotic analgesics Addiction & Tolerance Potential for abuse Acts on CNS More powerful analgesics Don’t reduce inflammation Preferred route is parenteral No addiction No abusive use Acts mainly on PNS Good analgesics Reduce inflammation Oral, parenteral route

Opiates: All the narcotic alkaloids found as natural product & its semi-synthetic derivatives Non-opiates: Synthetic narcotic compounds which may or may not be structural analogues of morphine Opioids: All the compounds which act on opioid receptors in the brain. It includes both opiates and non-opiates

Mechanism: Nociceptive pain arising from stimulation of peripheral pain receptors is relieved. Produce drowsiness without motor incoordination. Calming effect & loss of apprehension. Patients in pain or anxiety perceive these effects as pleasurable (euphoric effect), due to release of dopamine in brain. Morphine acts on µ-receptors in the brain to mediate its analgesic and sedative effect.

Classification 1. Natural opium alkaloids: Morphine sulphate, Codeine 2. Semi-synthetic derivatives (Opiates): Diacetyl morphine, Oxycodone, Hydrocodone, Oxymorphone 3. Synthetic derivatives (Non-opiates): Meperidine-HCl (Pethidine) Anileridine-HCl, Diphenoxylate-HCl, Loperamide-HCl, Fentanyl citrate, Methadone-HCl, Propoxyphene-HCl, Pentazocine, Levorphanol tartarate

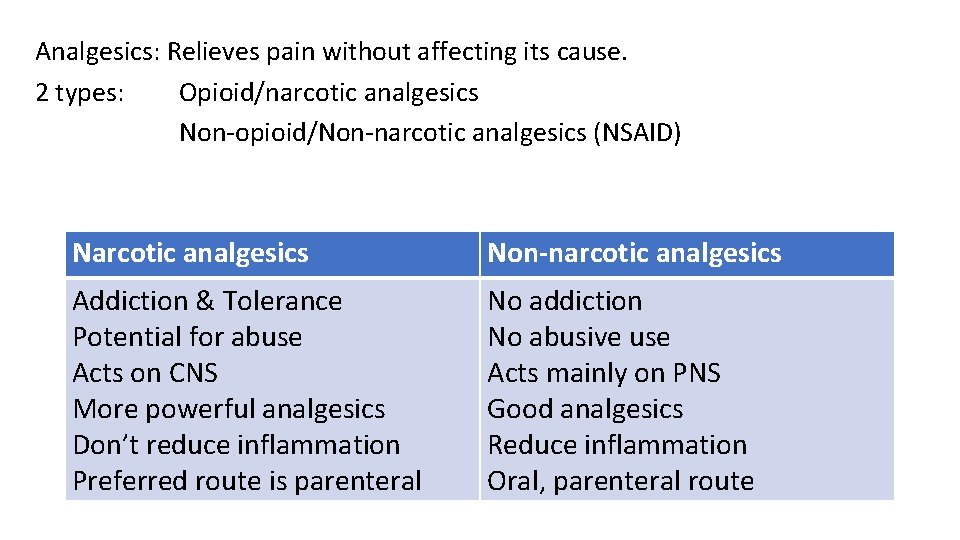
Morphine

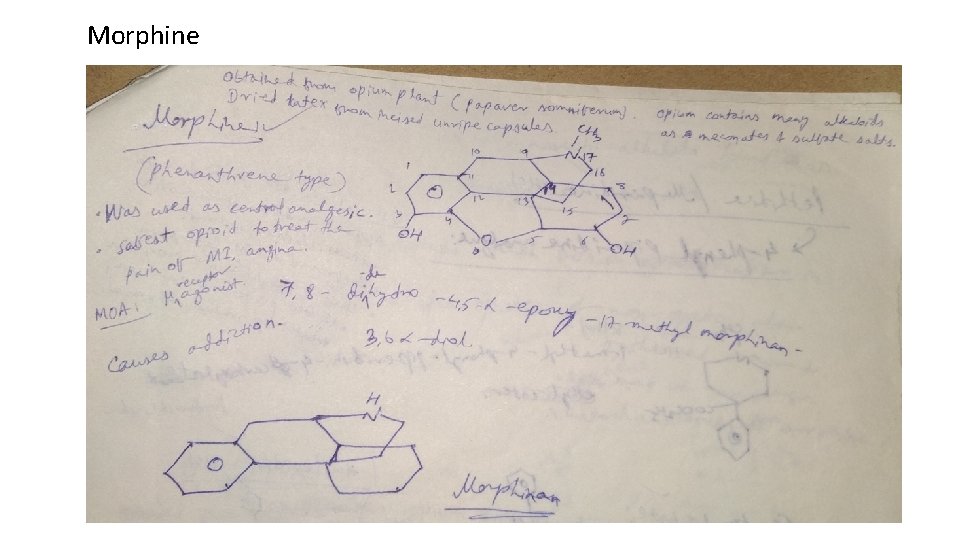
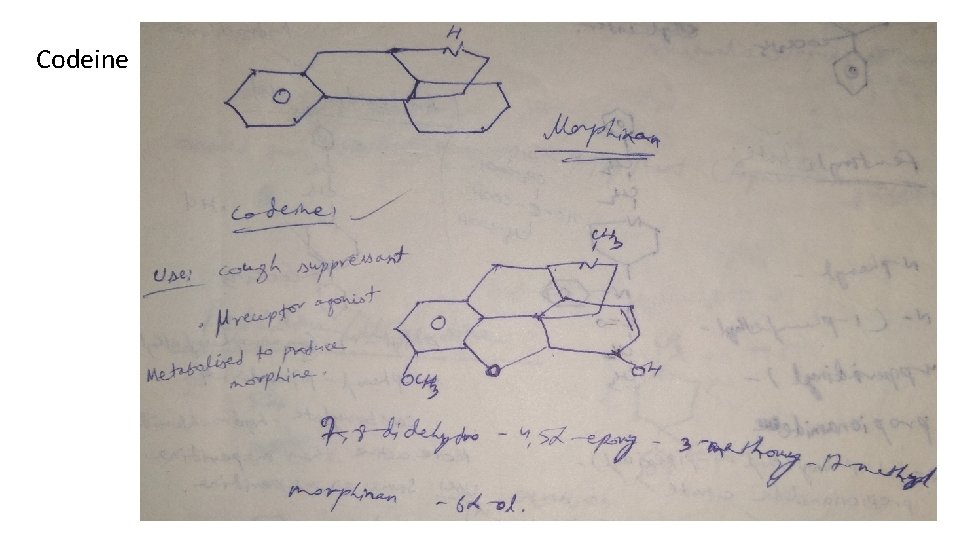
Codeine

Meperidine-HCl

Fentanyl citrate, Anileridine-HCl

Diphenoxylate-HCl Loperamide-HCl

Methadone-HCl

Propoxyphene-HCl

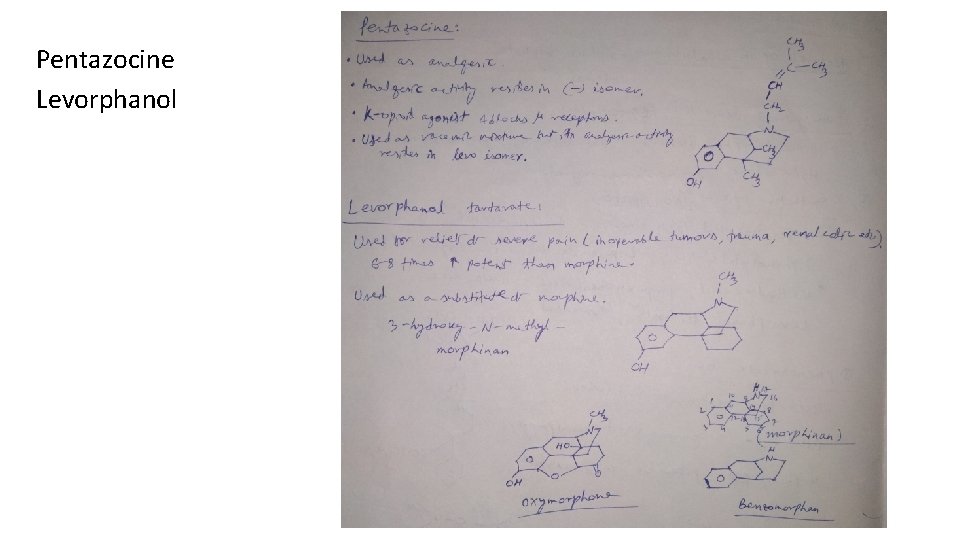
Pentazocine Levorphanol

Narcotic antagonists:

Levallorphan tartarate Naloxone-HCl

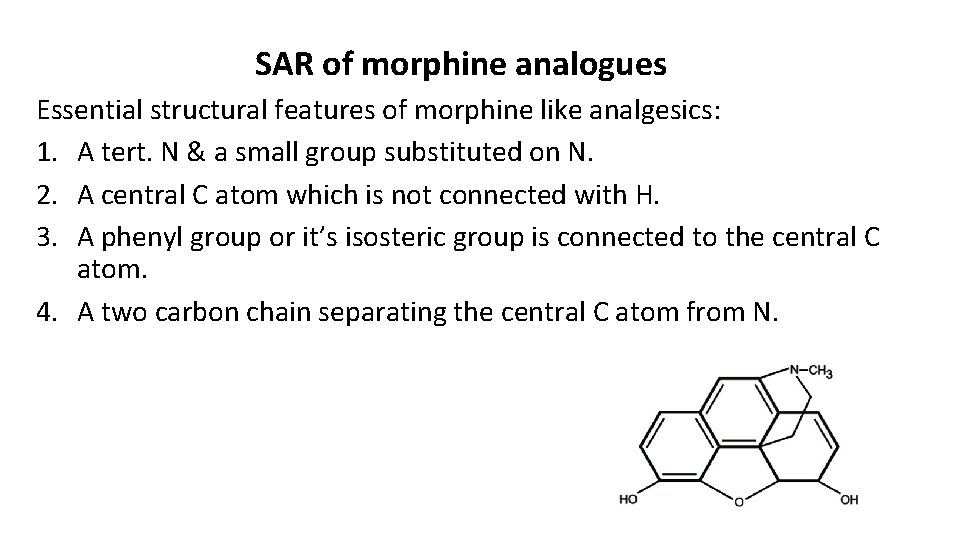
SAR of morphine analogues Essential structural features of morphine like analgesics: 1. A tert. N & a small group substituted on N. 2. A central C atom which is not connected with H. 3. A phenyl group or it’s isosteric group is connected to the central C atom. 4. A two carbon chain separating the central C atom from N.

Other features: 1. When phenolic 3 -OH is converted to methyl ether (codeine), analgesic activity becomes 1/10 th of morphine. 2. Esterification of 3 -OH: more active compounds 3. Acetylation of alcoholic 6 -OH: more active compounds 4. Inversion of 6 -OH group or its removal: more active analogues 5. Introduction of 14 -OH: more potent analgesic 6. N-methyl replaced by ethyl: activity decreases slightly N-methyl replaced by propyl, butyl, pentyl, hexyl, phenylethyl: increased Activity 7. N-allyl morphine (nalorphine): powerful morphine antagonist

8. Breaking of ether bridge & opening of piperidine ring: decreases activity 9. Hydrogenation of C 7 -C 8 double bond: equal or superior analgesic property 10. Substitution other than 3 rd position in the aromatic ring: reduce activity

END
 Srijoy mahapatra
Srijoy mahapatra Suchismit mahapatra
Suchismit mahapatra Analgesics examples
Analgesics examples Mechanism of action of opioid analgesics
Mechanism of action of opioid analgesics Classification of analgesic
Classification of analgesic Manoj mehta ficci
Manoj mehta ficci Manoj kuamr
Manoj kuamr Manoj abichandani
Manoj abichandani Aakriti went to her mother's brothers
Aakriti went to her mother's brothers Manoj radhakrishnan
Manoj radhakrishnan Manoj toshniwal dubai
Manoj toshniwal dubai Manoj chatlani
Manoj chatlani Manoj magician
Manoj magician Dr manoj das product
Dr manoj das product Mapa de colombia serrania de la macarena
Mapa de colombia serrania de la macarena Esgradinas
Esgradinas Meteorizacion
Meteorizacion Estructura de relieve ejemplos
Estructura de relieve ejemplos Astonosfera
Astonosfera Relieves emergidos y sumergidos
Relieves emergidos y sumergidos




































