Nanotechnology and Solar Energy Solar Electricity Photovoltaics Fuel





- Slides: 23

Nanotechnology and Solar Energy Solar Electricity • Photovoltaics Fuel from the Sun • Photosynthesis • Biofuels • Split Water • Fuel Cells Wed. Apr. 11, 2006 Phy 107 Lecture 30 1

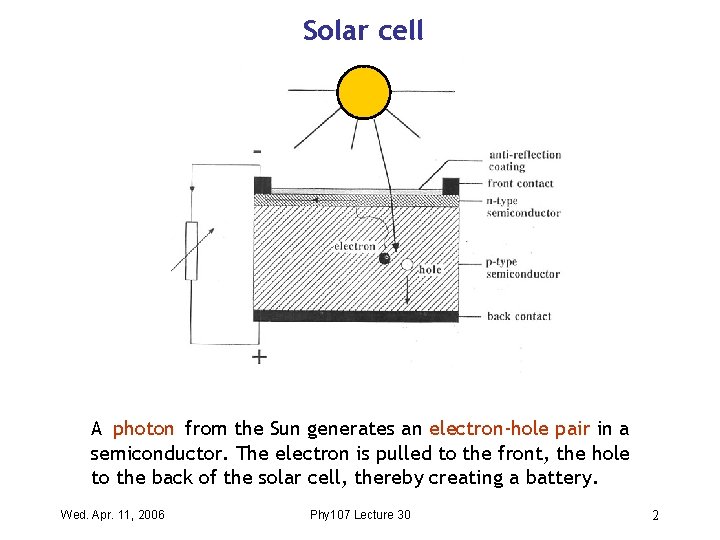
Solar cell A photon from the Sun generates an electron-hole pair in a semiconductor. The electron is pulled to the front, the hole to the back of the solar cell, thereby creating a battery. Wed. Apr. 11, 2006 Phy 107 Lecture 30 2

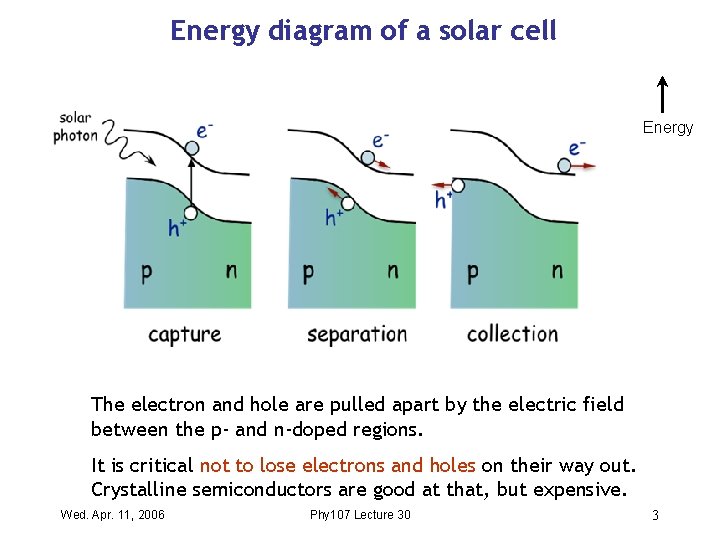
Energy diagram of a solar cell Energy The electron and hole are pulled apart by the electric field between the p- and n-doped regions. It is critical not to lose electrons and holes on their way out. Crystalline semiconductors are good at that, but expensive. Wed. Apr. 11, 2006 Phy 107 Lecture 30 3

Electrons, Holes, and Excitons Energy Electron Photon Band Gap Hole exciton A photon excites an electron across the band gap of a semiconductor. That leaves a hole among the occupied levels in the valence band an electron among the unoccupied levels in the conduction band. Electron and hole are attracted electrically and can form an exciton (similar to a hydrogen atom). In organic semiconductors it takes a significant amount of energy to. Phy 107 break them apart into free carriers. Wed. Apr. 11, 2006 Lecture 30 4

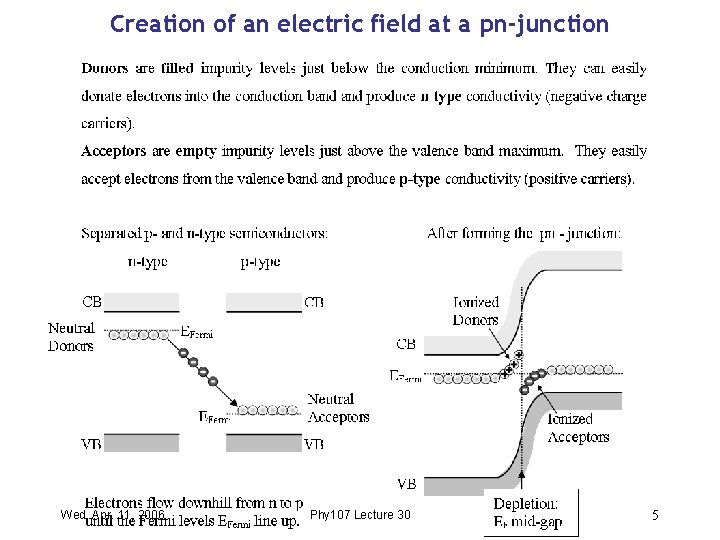
Creation of an electric field at a pn-junction Wed. Apr. 11, 2006 Phy 107 Lecture 30 5

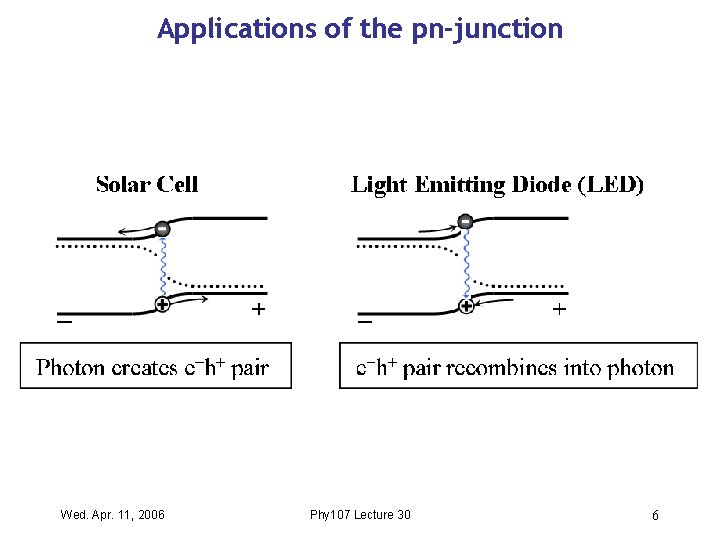
Applications of the pn-junction Wed. Apr. 11, 2006 Phy 107 Lecture 30 6

Many types of solar cells Wed. Apr. 11, 2006 Phy 107 Lecture 30 7

DOE Report http: //www. er. doe. gov/bes/reports/files/SEU_rpt. pdf Solar cells containing nanoparticles. Sensitized by a dye molecule similar to that on the cover. Wed. Apr. 11, 2006 Ti. O 2 nanoparticles act as acceptors, collecting electrons. The electrolyte acts 30 as donor, collecting the holes. Phy 107 Lecture 8

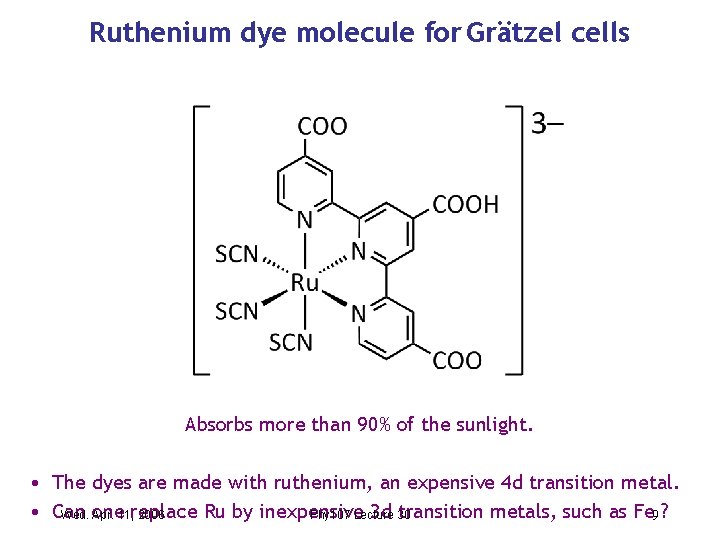
Ruthenium dye molecule for Grätzel cells Absorbs more than 90% of the sunlight. • The dyes are made with ruthenium, an expensive 4 d transition metal. • Can Ru by inexpensive 3 d 30 transition metals, such as Fe 9 ? Wed. one Apr. 11, replace 2006 Phy 107 Lecture

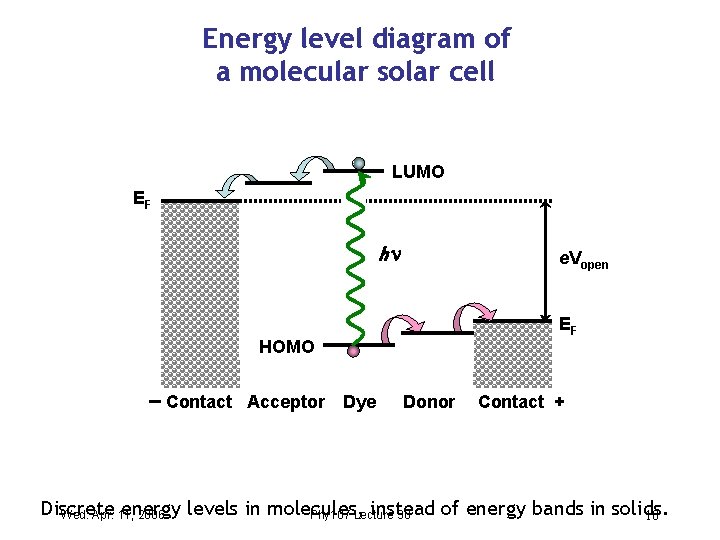
Energy level diagram of a molecular solar cell LUMO EF h e. Vopen EF HOMO Contact Acceptor Dye Donor Contact + Discrete energy levels in molecules, instead of energy bands in solids. Wed. Apr. 11, 2006 Phy 107 Lecture 30 10

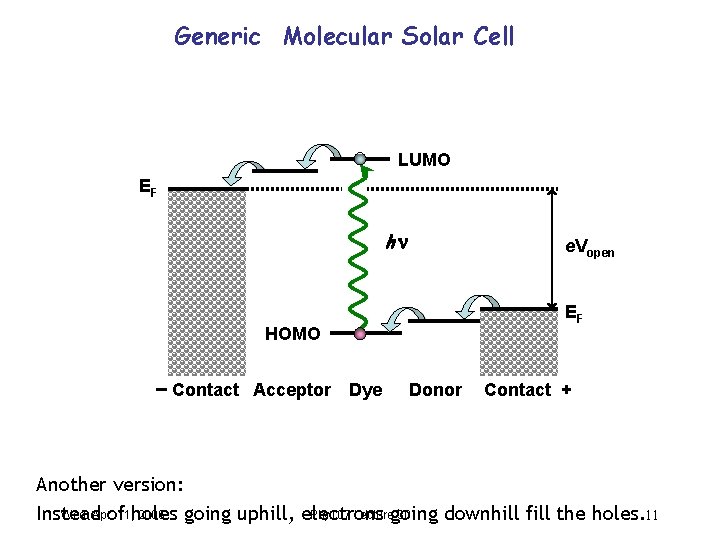
Generic Molecular Solar Cell LUMO EF h e. Vopen EF HOMO Contact Acceptor Dye Donor Contact + Another version: Wed. Apr. 2006 Phy 107 Lecturegoing 30 Instead of 11, holes going uphill, electrons downhill fill the holes. 11

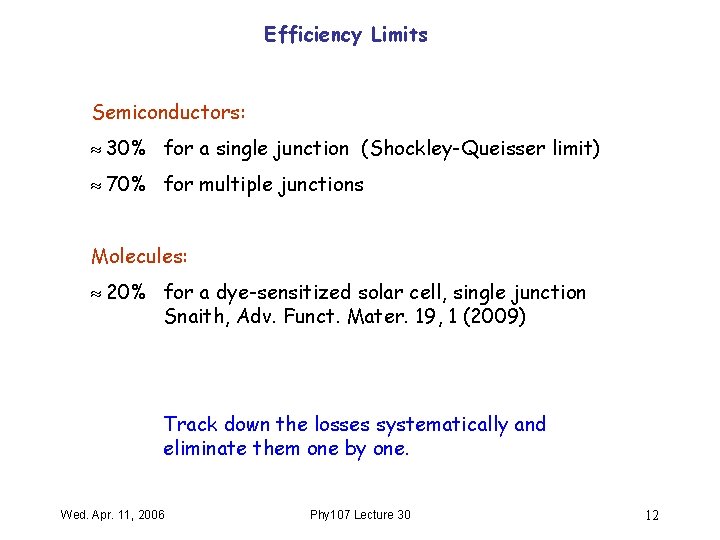
Efficiency Limits Semiconductors: 30% for a single junction (Shockley-Queisser limit) 70% for multiple junctions Molecules: 20% for a dye-sensitized solar cell, single junction Snaith, Adv. Funct. Mater. 19, 1 (2009) Track down the losses systematically and eliminate them one by one. Wed. Apr. 11, 2006 Phy 107 Lecture 30 12

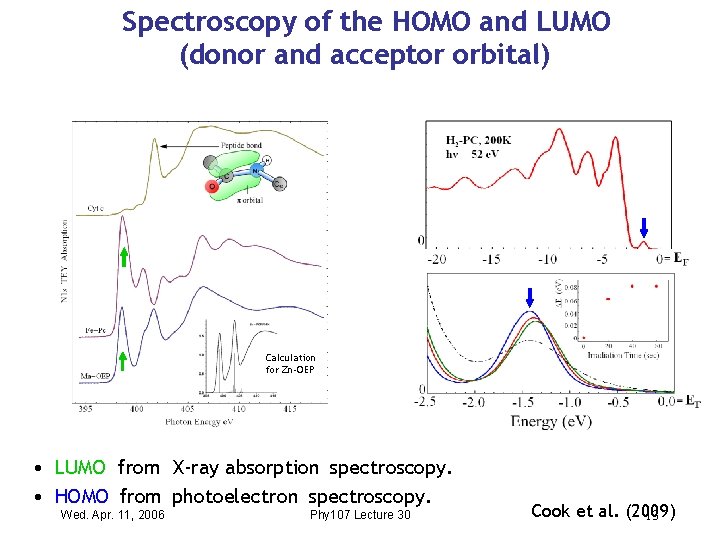
Spectroscopy of the HOMO and LUMO (donor and acceptor orbital) Calculation for Zn-OEP • LUMO from X-ray absorption spectroscopy. • HOMO from photoelectron spectroscopy. Wed. Apr. 11, 2006 Phy 107 Lecture 30 Cook et al. (2009) 13

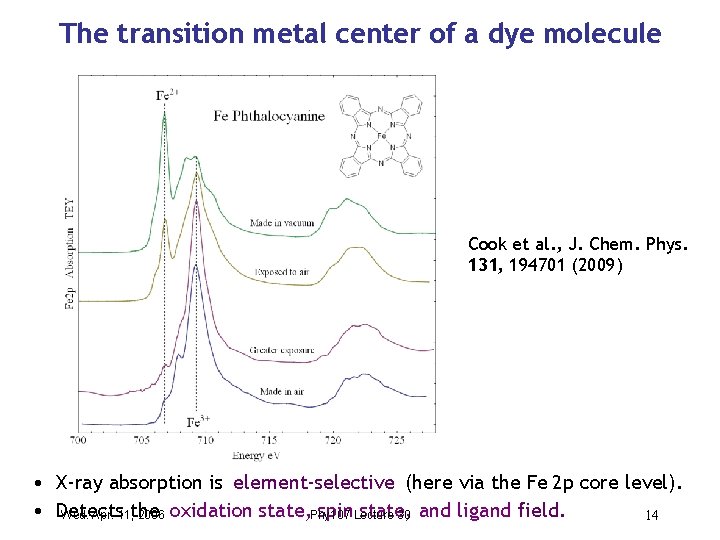
The transition metal center of a dye molecule Cook et al. , J. Chem. Phys. 131, 194701 (2009) • X-ray absorption is element-selective (here via the Fe 2 p core level). • Detects spin. Lecture state, Wed. Apr. 11, the 2006 oxidation state, Phy 107 30 and ligand field. 14

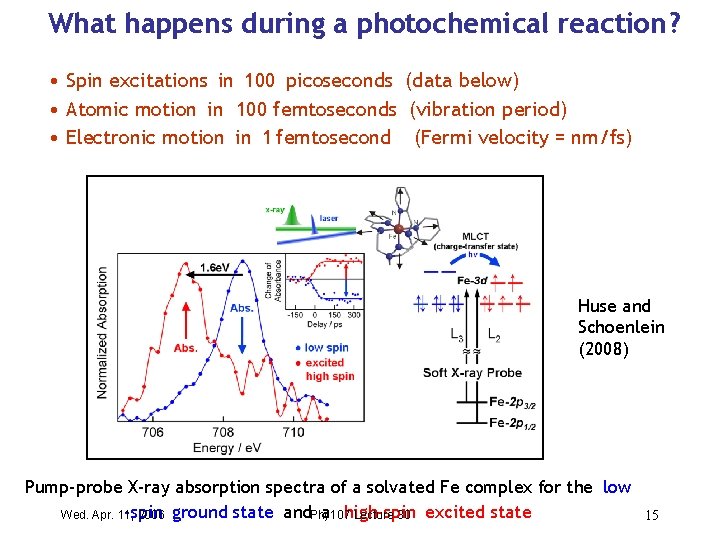
What happens during a photochemical reaction ? • Spin excitations in 100 picoseconds (data below) • Atomic motion in 100 femtoseconds (vibration period) • Electronic motion in 1 femtosecond (Fermi velocity = nm/fs) Huse and Schoenlein (2008) Pump-probe X-ray absorption spectra of a solvated Fe complex for the low -spin a high-spin Wed. Apr. 11, 2006 ground state and. Phy 107 Lecture 30 excited state 15

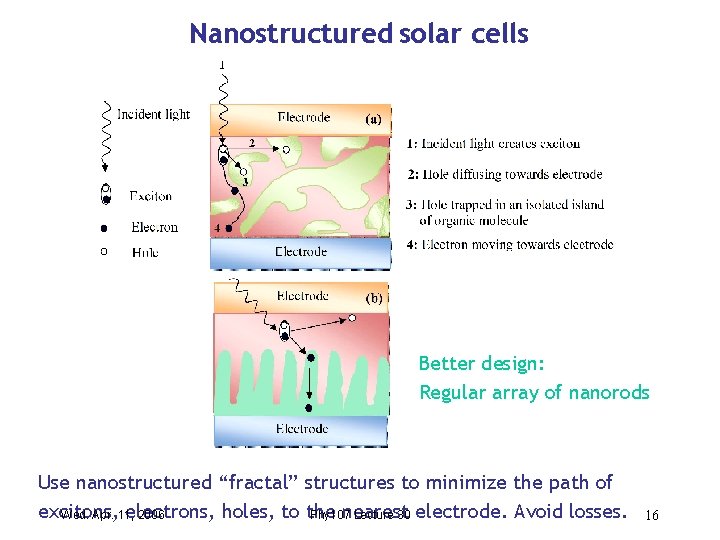
Nanostructured solar cells Better design: Regular array of nanorods Use nanostructured “fractal” structures to minimize the path of excitons, electrons, holes, to the nearest Wed. Apr. 11, 2006 Phy 107 Lecture 30 electrode. Avoid losses. 16

Zn. O nanorods as electrode Growth time increases from left to right. (a)-(c) side view (500 nm bar), (d)-(f) top view (100 nm bar). Wed. Apr. 11, 2006 Phy 107 Lecture 30 17 Baxter et al. , Nanotechnology 17, S 304 (2006) and Appl. Phys. Lett. 86, 053114 (2005).

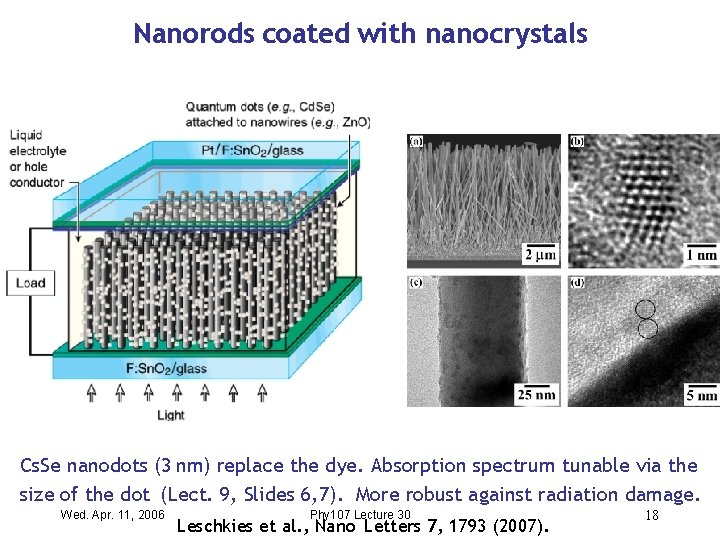
Nanorods coated with nanocrystals Cs. Se nanodots (3 nm) replace the dye. Absorption spectrum tunable via the size of the dot (Lect. 9, Slides 6, 7). More robust against radiation damage. Wed. Apr. 11, 2006 Phy 107 Lecture 30 Leschkies et al. , Nano Letters 7, 1793 (2007). 18

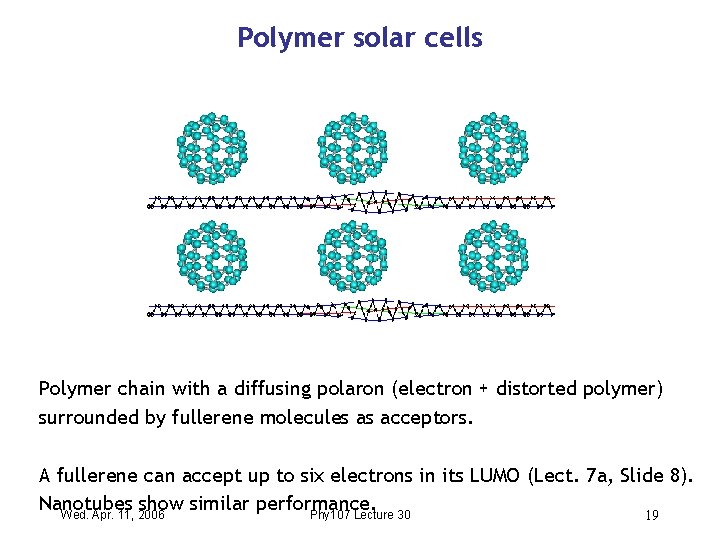
Polymer solar cells Polymer chain with a diffusing polaron (electron + distorted polymer) surrounded by fullerene molecules as acceptors. A fullerene can accept up to six electrons in its LUMO (Lect. 7 a, Slide 8). Nanotubes show similar performance. Wed. Apr. 11, 2006 Phy 107 Lecture 30 19

Fuel from the Sun • Photosynthesis How does nature convert solar energy to chemical energy ? • Biofuels Convert plants into fuel: Make ethanol, diesel fuel from sugar, corn starch, plant oil, cellulose, algae, . . . • Split Water Split water into hydrogen and oxygen using sunlight. Use hydrogen as fuel. No greenhouse gases. • Fuel Cells Producing electricity directly from fuel and oxygen. Wed. Apr. 11, 2006 Phy 107 Lecture 30 20

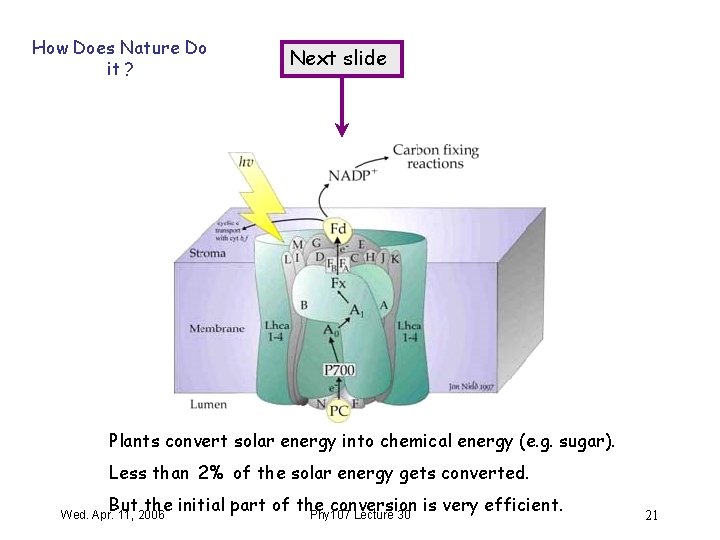
How Does Nature Do it ? Next slide Plants convert solar energy into chemical energy (e. g. sugar). Less than 2% of the solar energy gets converted. But the initial part of the conversion is very efficient. Phy 107 Lecture 30 Wed. Apr. 11, 2006 21

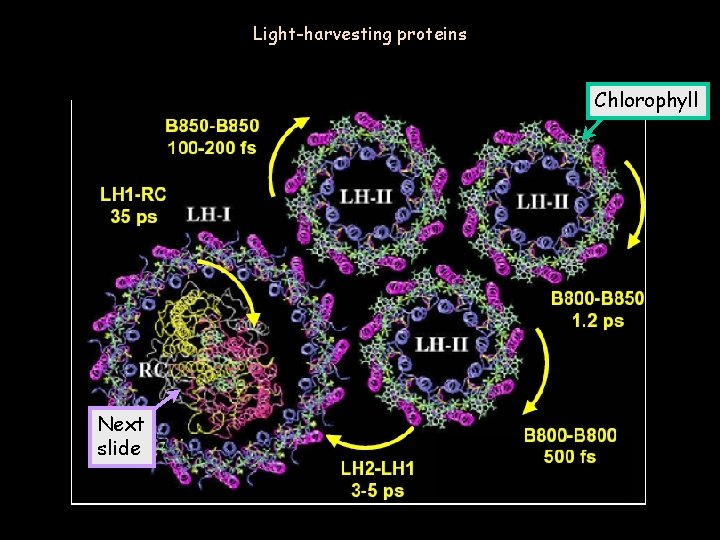
Light-harvesting proteins Chlorophyll Next slide Wed. Apr. 11, 2006 Phy 107 Lecture 30 22

The Oxygen Evolving Complex 4 Mn + 1 Ca Instead of rare Pt (5 d), Rh (4 d), nature uses plentiful Mn (3 d), Fe (3 d), Ca(3 d) ascatalysts. Can we do that in artificial photosynthesis ? What does it take ? (3 D cage? ) Wed. Apr. 11, 2006 Phy 107 Lecture 30 23
 Static electricity and current electricity
Static electricity and current electricity Static electricity and current electricity
Static electricity and current electricity Electricity and magnetism vocabulary
Electricity and magnetism vocabulary What is an inexhaustible source of energy
What is an inexhaustible source of energy Advantages and disadvantages of nanotechnology
Advantages and disadvantages of nanotechnology Difference between nanoscience and nanotechnology
Difference between nanoscience and nanotechnology Journal of nanoscience and nanotechnology sci
Journal of nanoscience and nanotechnology sci Fossil
Fossil Pros and cons of fossil fuels
Pros and cons of fossil fuels Indirect forms of solar energy
Indirect forms of solar energy Timeline of nanotechnology
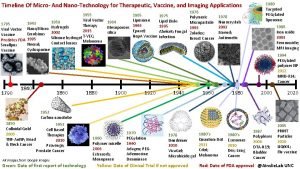
Timeline of nanotechnology What is nanotechnology
What is nanotechnology Nanotechnology for kids
Nanotechnology for kids Nanotechnology in sports
Nanotechnology in sports Nanotechnology fact or myth articles
Nanotechnology fact or myth articles Disadvantages of nanotechnology
Disadvantages of nanotechnology Nanotechnology
Nanotechnology What is nanotechnology
What is nanotechnology Conclusion of nanotechnology
Conclusion of nanotechnology Interpretations of more’s law assert that
Interpretations of more’s law assert that Plasma arcing in nanotechnology
Plasma arcing in nanotechnology Nanotechnology definition
Nanotechnology definition What is nanotechnology
What is nanotechnology Nanotechnology
Nanotechnology