Nanoparticles as a formulation platform for bioavailabilitychallenged molecules
Nanoparticles as a formulation platform for bioavailabilitychallenged molecules; Highlights on the benefits of the leon Technology, characterization and rapid scale up from bench to clinic Pascale Clement, Ph. D Formulation & Delivery Series, 8 -9 July 2020
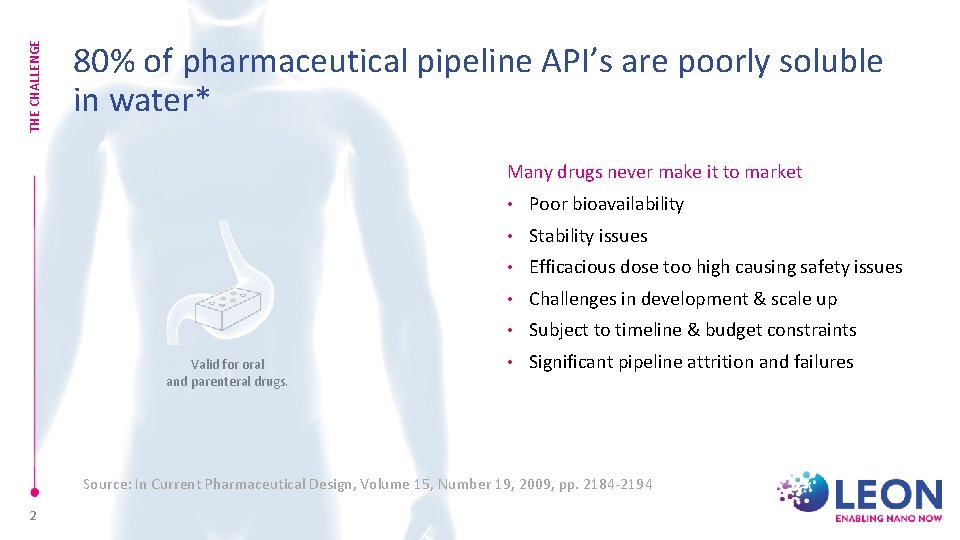
THE CHALLENGE 80% of pharmaceutical pipeline API’s are poorly soluble in water* Many drugs never make it to market Valid for oral and parenteral drugs. • Poor bioavailability • Stability issues • Efficacious dose too high causing safety issues • Challenges in development & scale up • Subject to timeline & budget constraints • Significant pipeline attrition and failures Source: In Current Pharmaceutical Design, Volume 15, Number 19, 2009, pp. 2184 -2194 2
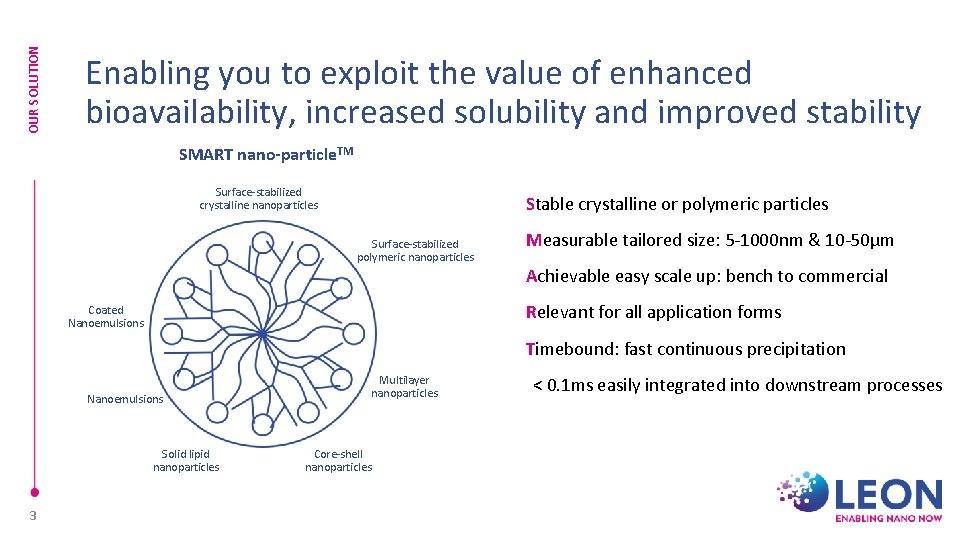
OUR SOLUTION Enabling you to exploit the value of enhanced bioavailability, increased solubility and improved stability SMART nano-particle. TM Surface-stabilized crystalline nanoparticles Stable crystalline or polymeric particles Surface-stabilized polymeric nanoparticles Measurable tailored size: 5 -1000 nm & 10 -50µm Achievable easy scale up: bench to commercial Relevant for all application forms Coated Nanoemulsions Timebound: fast continuous precipitation Nanoemulsions Solid lipid nanoparticles 3 Multilayer nanoparticles Core-shell nanoparticles < 0. 1 ms easily integrated into downstream processes
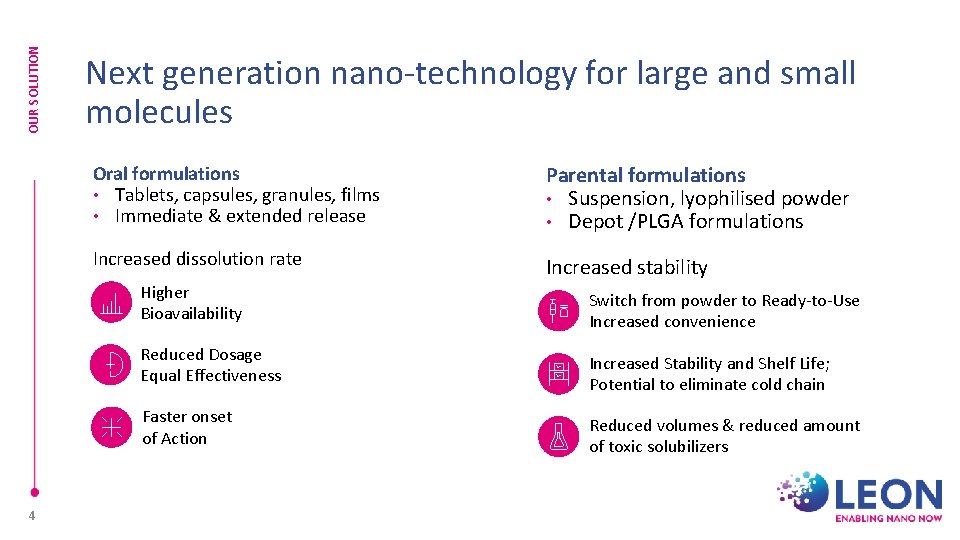
OUR SOLUTION Next generation nano-technology for large and small molecules Oral formulations • Tablets, capsules, granules, films • Immediate & extended release Parental formulations • Suspension, lyophilised powder • Depot /PLGA formulations Increased dissolution rate Increased stability Higher Bioavailability Reduced Dosage Equal Effectiveness Faster onset of Action 4 Switch from powder to Ready-to-Use Increased convenience Increased Stability and Shelf Life; Potential to eliminate cold chain Reduced volumes & reduced amount of toxic solubilizers
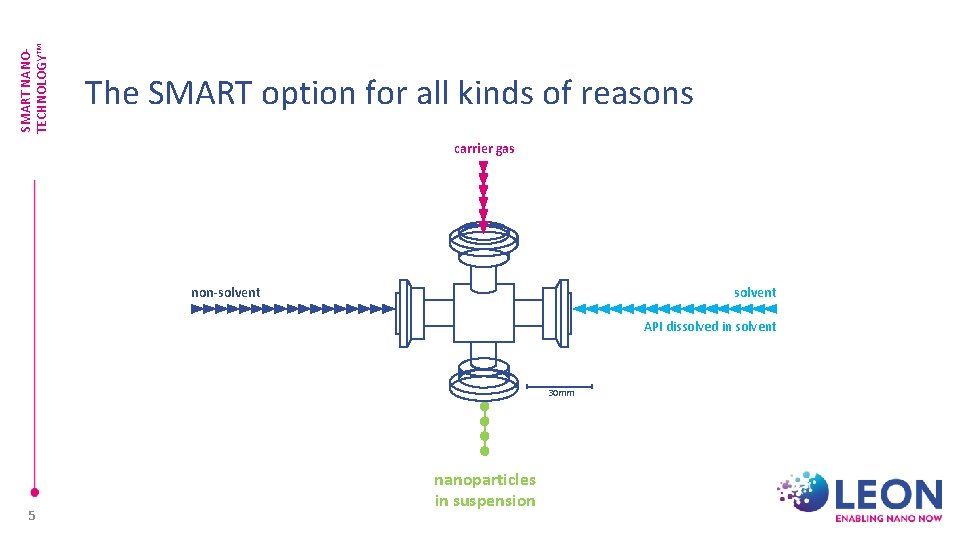
SMART NANOTECHNOLOGY™ The SMART option for all kinds of reasons carrier gas non-solvent API dissolved in solvent 30 mm 5 nanoparticles in suspension
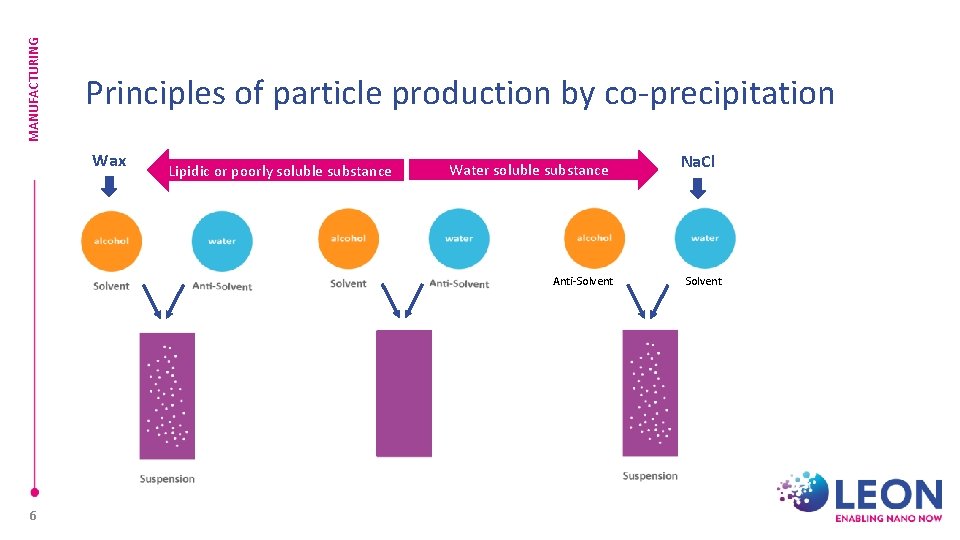
MANUFACTURING Principles of particle production by co-precipitation Wax Lipidic or poorly soluble substance Water soluble substance Anti-Solvent 6 Na. Cl Solvent
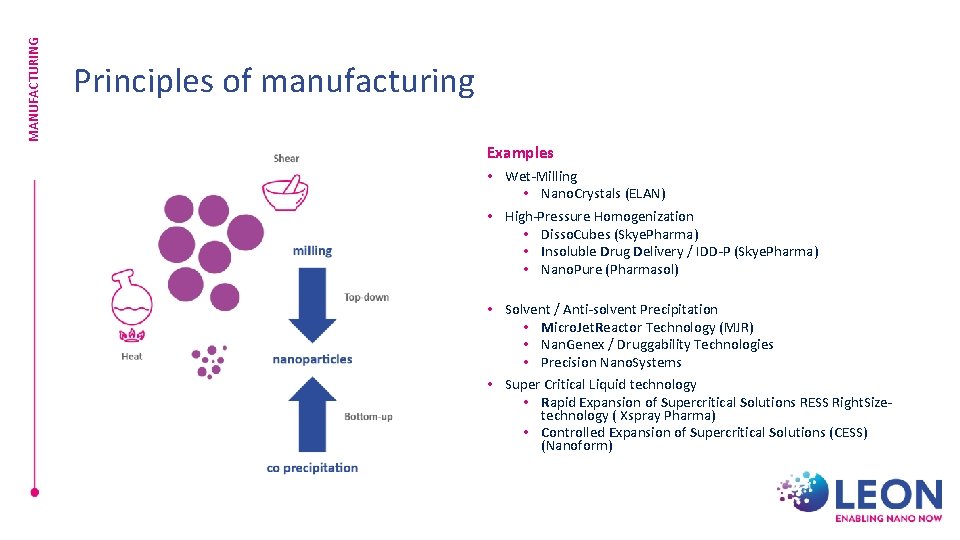
MANUFACTURING Principles of manufacturing Examples • Wet-Milling • Nano. Crystals (ELAN) • High-Pressure Homogenization • Disso. Cubes (Skye. Pharma) • Insoluble Drug Delivery / IDD-P (Skye. Pharma) • Nano. Pure (Pharmasol) • Solvent / Anti-solvent Precipitation • Micro. Jet. Reactor Technology (MJR) • Nan. Genex / Druggability Technologies • Precision Nano. Systems • Super Critical Liquid technology • Rapid Expansion of Supercritical Solutions RESS Right. Sizetechnology ( Xspray Pharma) • Controlled Expansion of Supercritical Solutions (CESS) (Nanoform)
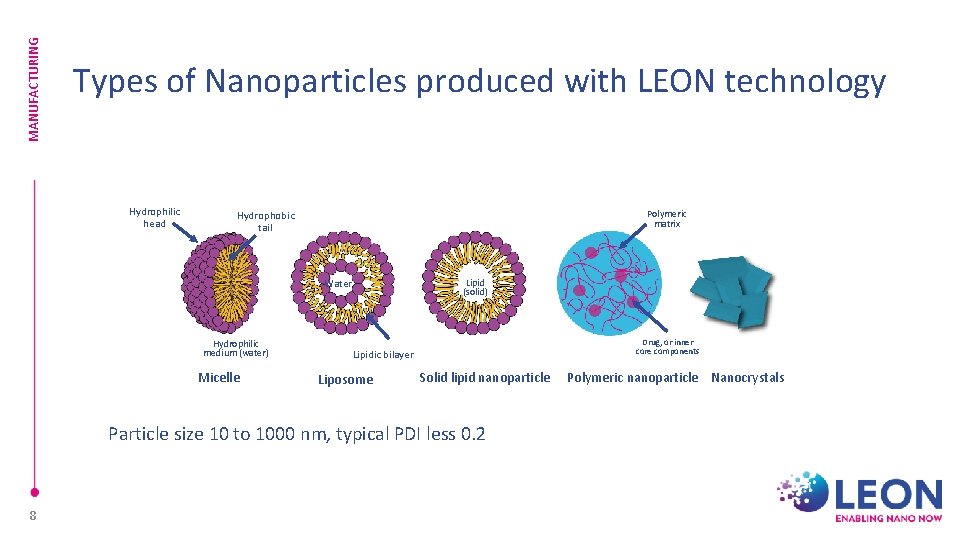
MANUFACTURING Types of Nanoparticles produced with LEON technology Hydrophilic head Polymeric matrix Hydrophobic tail Lipid (solid) Water Hydrophilic medium (water) Micelle Drug, or inner core components Lipidic bilayer Liposome Solid lipid nanoparticle Particle size 10 to 1000 nm, typical PDI less 0. 2 8 Polymeric nanoparticle Nanocrystals
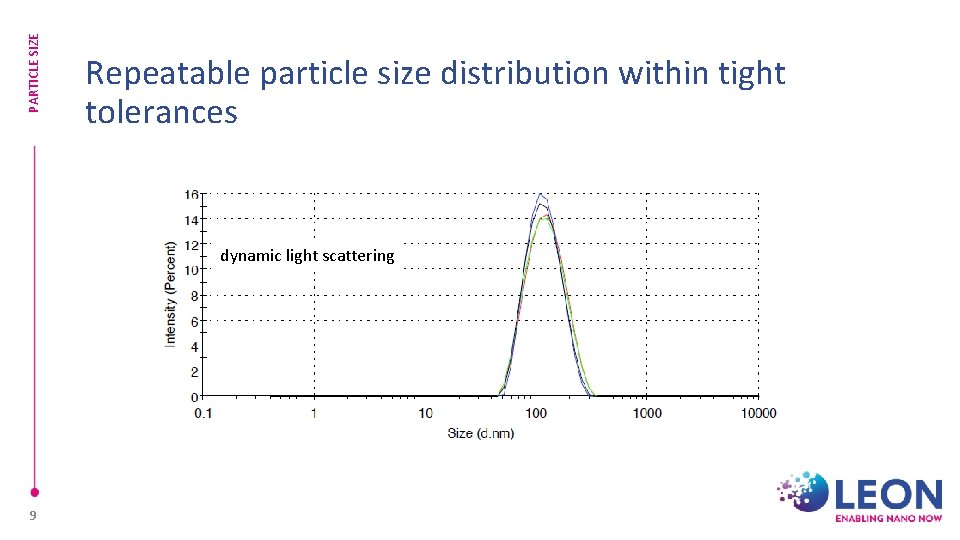
PARTICLE SIZE Repeatable particle size distribution within tight tolerances dynamic light scattering 9
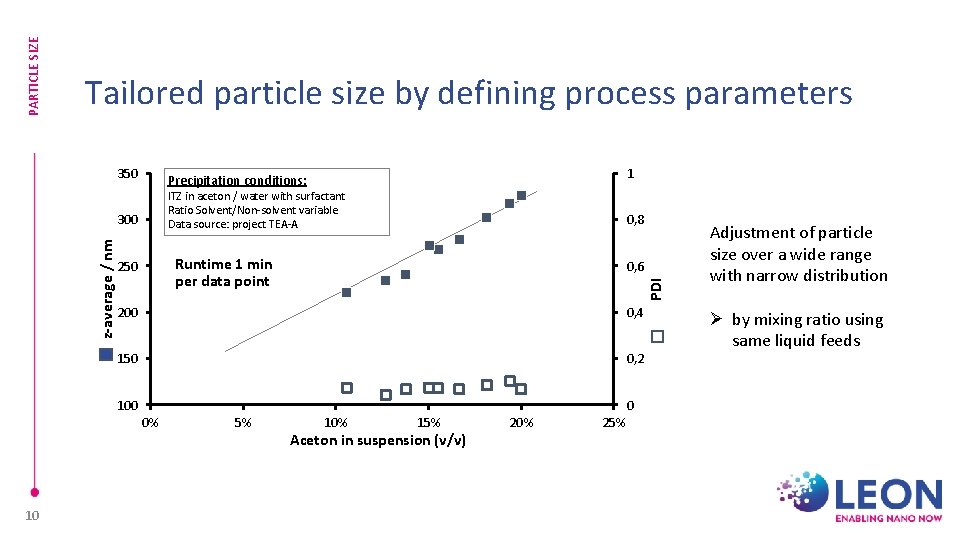
PARTICLE SIZE Tailored particle size by defining process parameters 350 1 Precipitation conditions: ITZ in aceton / water with surfactant Ratio Solvent/Non-solvent variable Data source: project TEA-A with water Runtime 1 min per data point 250 0, 6 200 0, 4 150 0, 2 100 0 0% 10 0, 8 PDI z-average / nm 300 Diluted 10 µl at 1000 µl 5% 10% 15% Aceton in suspension (v/v) 20% 25% Adjustment of particle size over a wide range with narrow distribution Ø by mixing ratio using same liquid feeds
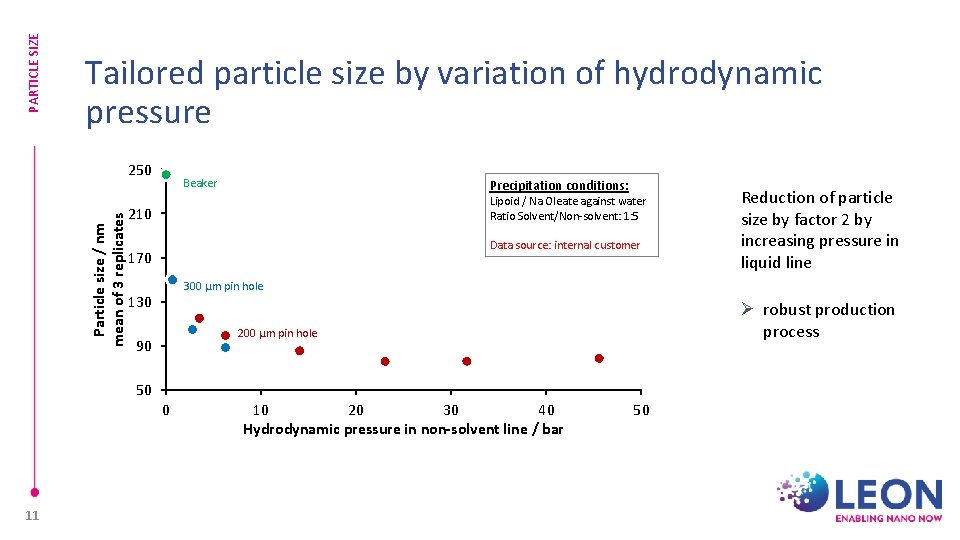
PARTICLE SIZE Tailored particle size by variation of hydrodynamic pressure Particle size / nm mean of 3 replicates 250 Beaker Precipitation conditions: Lipoid / Na Oleate against water Ratio Solvent/Non-solvent: 1: 5 210 Data source: internal customer 170 300 µm pin hole 130 Ø robust production process 200 µm pin hole 90 50 0 11 Reduction of particle size by factor 2 by increasing pressure in liquid line 10 20 30 40 Hydrodynamic pressure in non-solvent line / bar 50
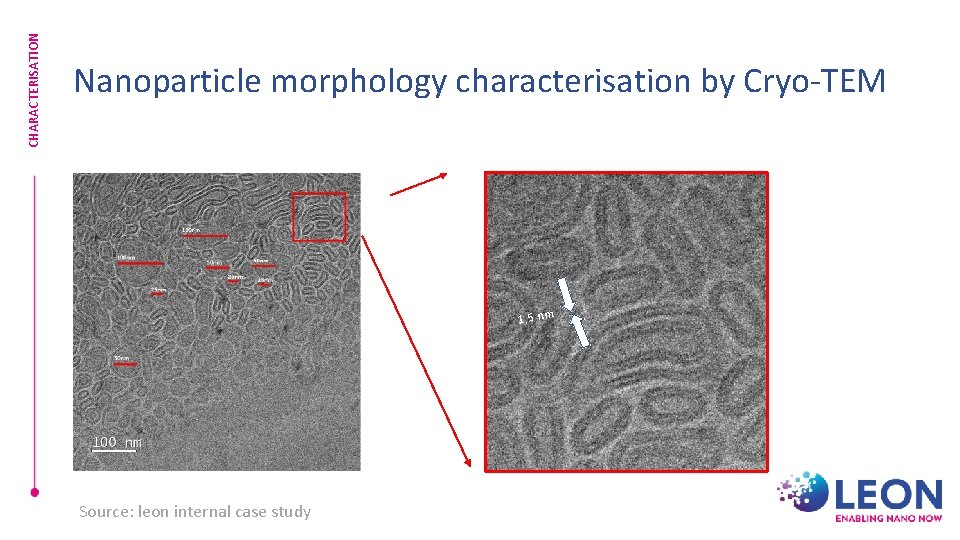
CHARACTERISATION Nanoparticle morphology characterisation by Cryo-TEM 1, 5 nm Source: leon internal case study
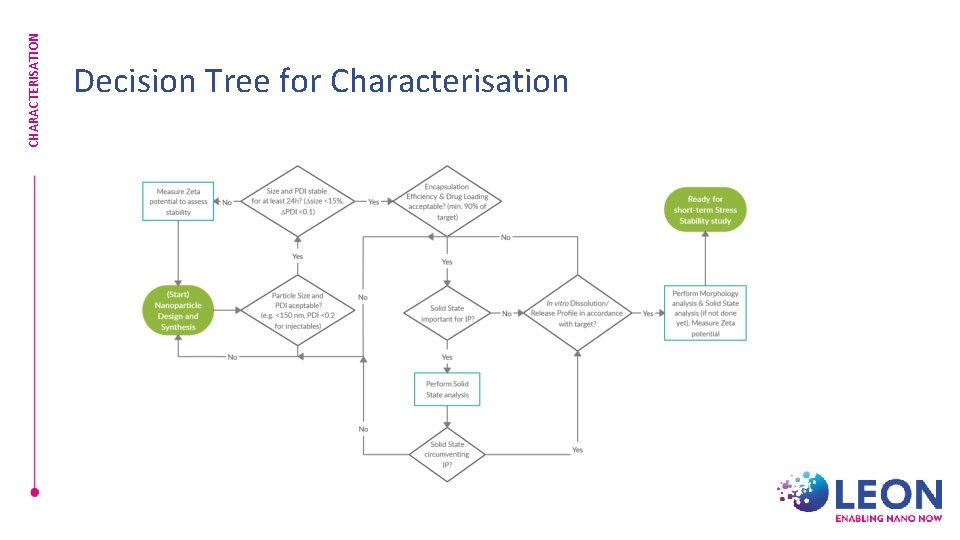
CHARACTERISATION Decision Tree for Characterisation
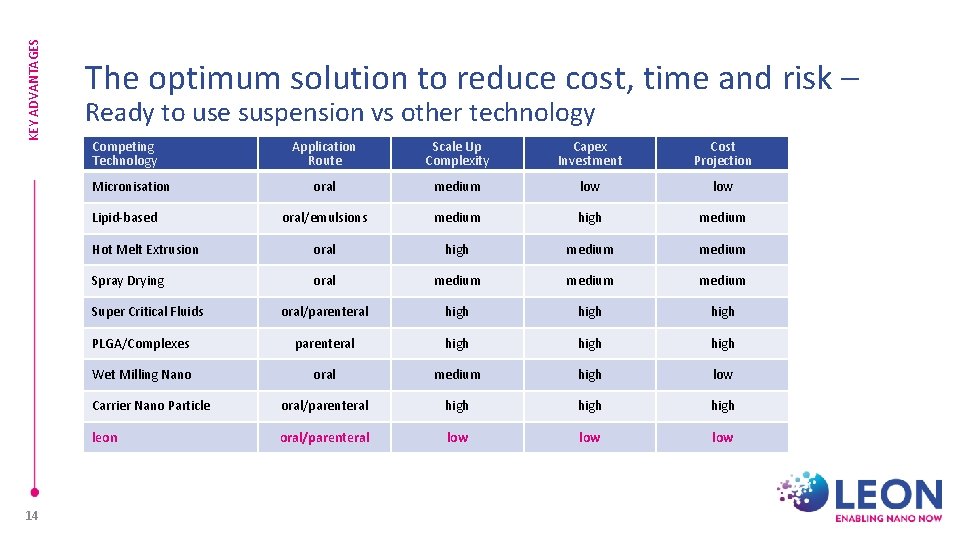
KEY ADVANTAGES The optimum solution to reduce cost, time and risk – Ready to use suspension vs other technology Competing Technology Application Route Scale Up Complexity Capex Investment Cost Projection oral medium low oral/emulsions medium high medium Hot Melt Extrusion oral high medium Spray Drying oral medium oral/parenteral high PLGA/Complexes parenteral high Wet Milling Nano oral medium high low Carrier Nano Particle oral/parenteral high leon oral/parenteral low low Micronisation Lipid-based Super Critical Fluids 14
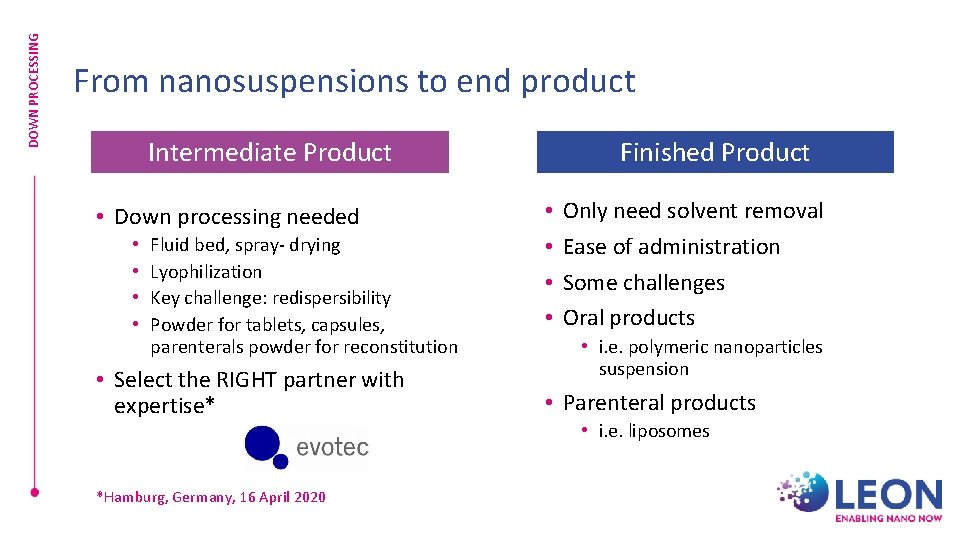
DOWN PROCESSING From nanosuspensions to end product Intermediate Product • Down processing needed • • Fluid bed, spray- drying Lyophilization Key challenge: redispersibility Powder for tablets, capsules, parenterals powder for reconstitution • Select the RIGHT partner with expertise* *Hamburg, Germany, 16 April 2020 Finished Product • • Only need solvent removal Ease of administration Some challenges Oral products • i. e. polymeric nanoparticles suspension • Parenteral products • i. e. liposomes
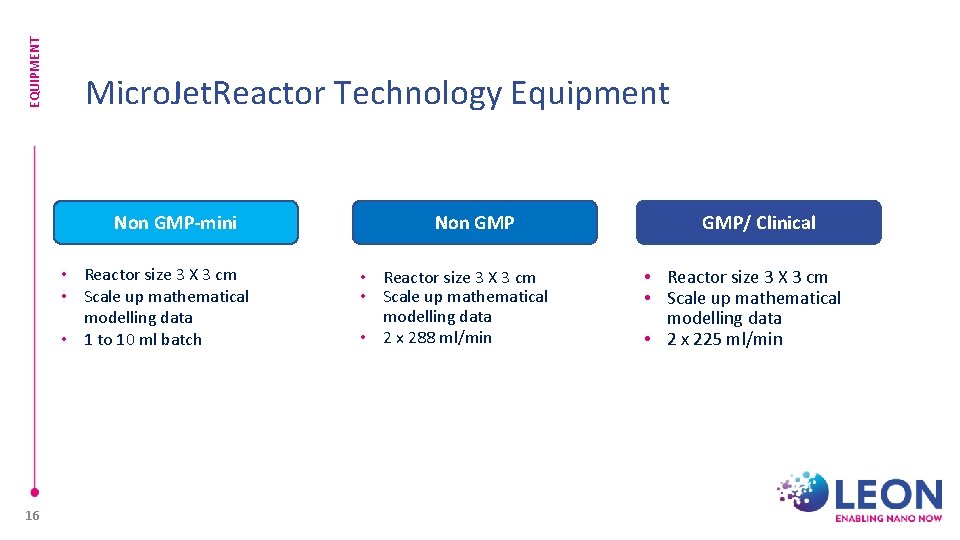
EQUIPMENT Micro. Jet. Reactor Technology Equipment Non GMP-mini • Reactor size 3 X 3 cm • Scale up mathematical modelling data • 1 to 10 ml batch 16 Non GMP • Reactor size 3 X 3 cm • Scale up mathematical modelling data • 2 x 288 ml/min GMP/ Clinical • Reactor size 3 X 3 cm • Scale up mathematical modelling data • 2 x 225 ml/min
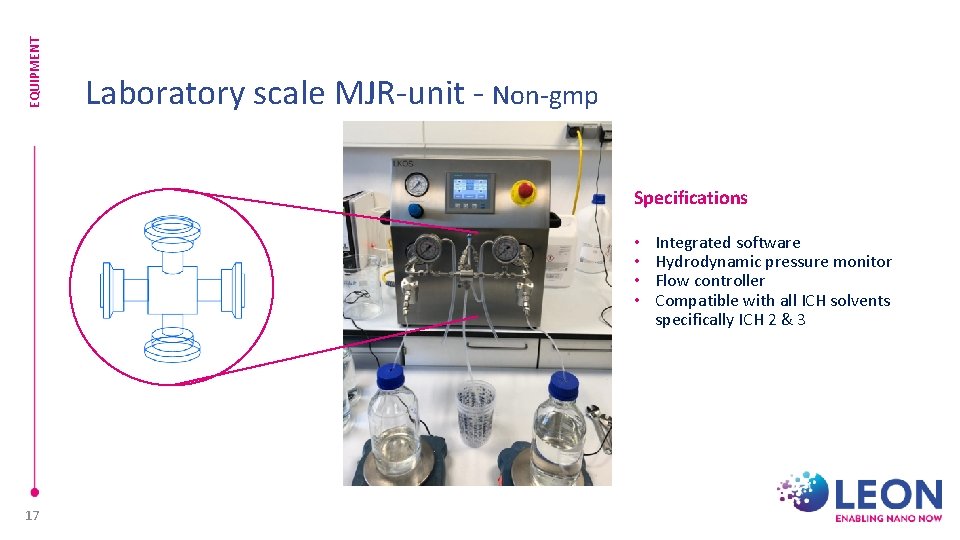
EQUIPMENT Laboratory scale MJR-unit - Non-gmp Specifications • • 17 Integrated software Hydrodynamic pressure monitor Flow controller Compatible with all ICH solvents specifically ICH 2 & 3
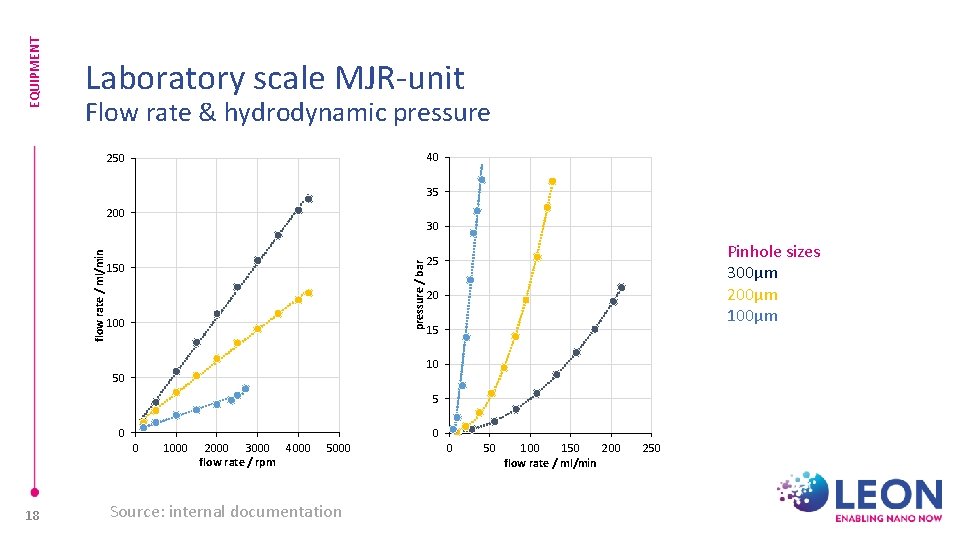
EQUIPMENT Laboratory scale MJR-unit Flow rate & hydrodynamic pressure 40 250 35 30 pressure / bar flow rate / ml/min 200 150 100 Pinhole sizes 300µm 200µm 100µm 25 20 15 10 50 5 0 0 18 1000 2000 3000 4000 flow rate / rpm 5000 Source: internal documentation 0 0 50 100 150 200 flow rate / ml/min 250
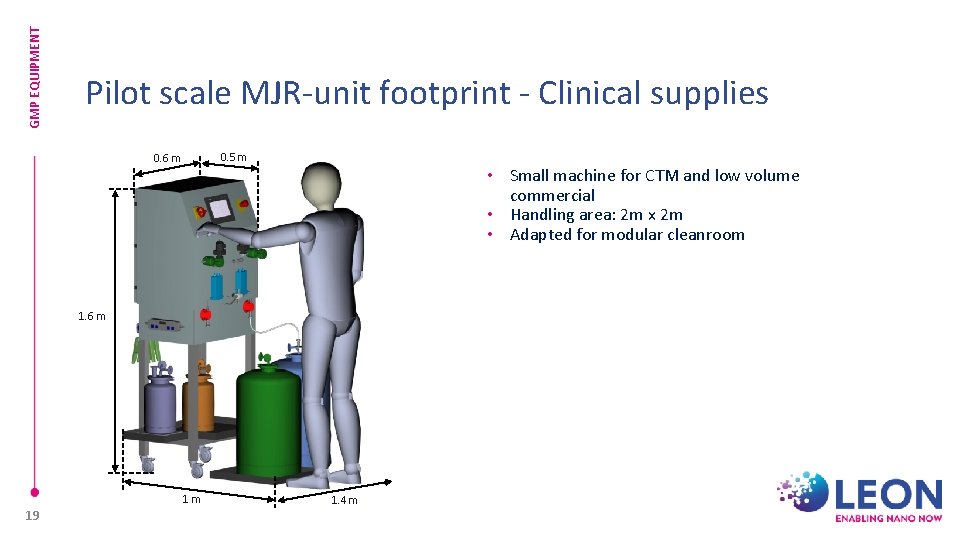
GMP EQUIPMENT Pilot scale MJR-unit footprint - Clinical supplies 0. 5 m 0. 6 m • Small machine for CTM and low volume commercial • Handling area: 2 m x 2 m • Adapted for modular cleanroom 1. 6 m 1 m 19 1. 4 m
WHY LEON Enabling efficacy, safety and adherence, now Improved bioavailability and faster onset of action • Dose reduction resulting in lower drug exposure • Improved stability • Extension of product life cycle • Faster to market • Lower pill burden • Eliminates food effect • Increases patient compliance • • 20 3 -year goal from concept to FDA / EMA submission due to abbreviated regulatory pathway, e. g. 505(b)2, Hybrid Application

- Slides: 21