Naming the invisible Nomenclature and notation 1 Nomenclature

Naming the invisible Nomenclature and notation

1. Nomenclature a) Definition: – System of official names given to chemical compounds in the field of science • E. g. carbon dioxide sodium chloride

Nomenclature b) Steps for binary covalent molecules For naming molecules made up of 2 types of nonmetals – 1. name the 1 st element – 2. change the name of the second element to end in ide – 3. Add, if needed, a prefix or prefixes to specify the number of atoms of each element

Nomenclature • Step 3 (Continued) – List of prefixes • • • 2 3 4 5 6 7 di tri tetra penta hexa octa

• For example… • CO 2 – Made up of two types of non-metals (carbon and oxygen) – Name the 1 st element: carbon – Change the 2 nd element: oxygen → oxide – Add the prefixes: there are 2 oxygen atoms The prefix for 2 is di CARBON DIOXIDE
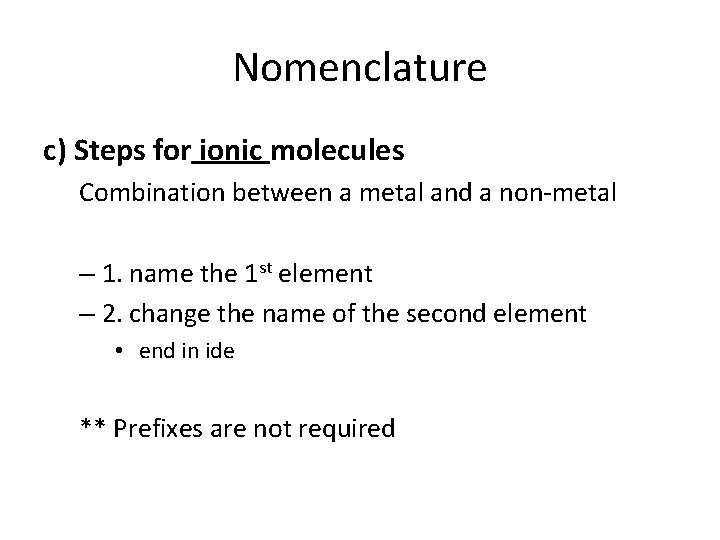
Nomenclature c) Steps for ionic molecules Combination between a metal and a non-metal – 1. name the 1 st element – 2. change the name of the second element • end in ide ** Prefixes are not required

• For example… • Na. Cl – Made up of a metal and a non-metal (sodium and chlorine) – Name the 1 st element: Sodium – Change the 2 nd element: chlorine → chloride SODIUM CHLORIDE
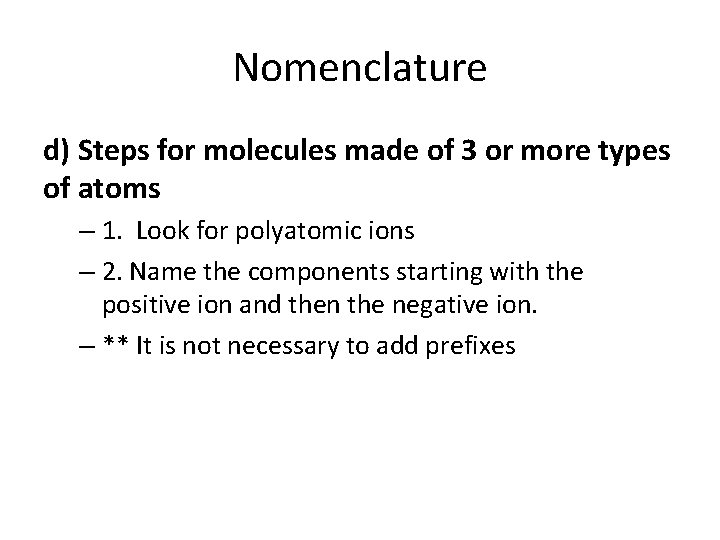
Nomenclature d) Steps for molecules made of 3 or more types of atoms – 1. Look for polyatomic ions – 2. Name the components starting with the positive ion and then the negative ion. – ** It is not necessary to add prefixes

• For example… • KOH – Made up of three different elements (sodium and chlorine) – Find the polyatomic ion (OH-) – Name the 1 st element: Potassium – Name the polyatomic ion: Hydroxide POTASSIUM HYDROXIDE

What is all this talk about ions 2. An Ion: – Atom with more or less electron than usual, therefore that is electrically charged – Will have an ionic charge based on its valence electrons 3. A Polyatomic Ion: – A grouping of elements that are not yet stable. They are chemically bound together yet still possess a charge.

4. Notation • Definition: – Chemical notation (chemical formulas) is a way to express information about the composition of a compound • E. g. H 2 O C 6 H 12 O 6
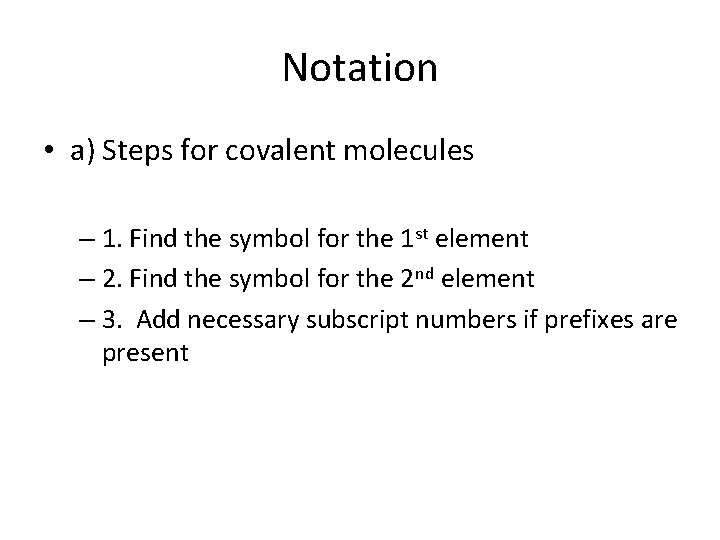
Notation • a) Steps for covalent molecules – 1. Find the symbol for the 1 st element – 2. Find the symbol for the 2 nd element – 3. Add necessary subscript numbers if prefixes are present
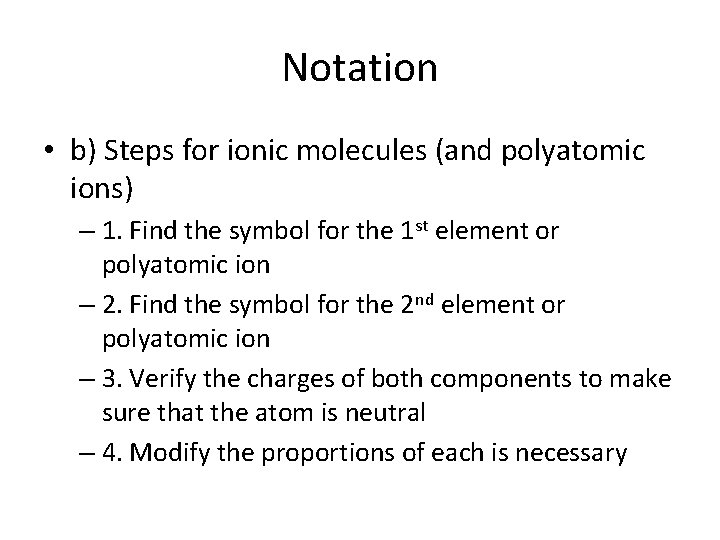
Notation • b) Steps for ionic molecules (and polyatomic ions) – 1. Find the symbol for the 1 st element or polyatomic ion – 2. Find the symbol for the 2 nd element or polyatomic ion – 3. Verify the charges of both components to make sure that the atom is neutral – 4. Modify the proportions of each is necessary
- Slides: 13