Naming Multivalent Compounds What are multivalent ions Some






- Slides: 8

Naming Multivalent Compounds

What are multivalent ions? • Some transition metals can form more than one ion • In other words some have more than 1 ion form For Example: Copper has 2 ion forms Copper I Chloride Can be a 1+ or 2+ ion Copper II Chloride

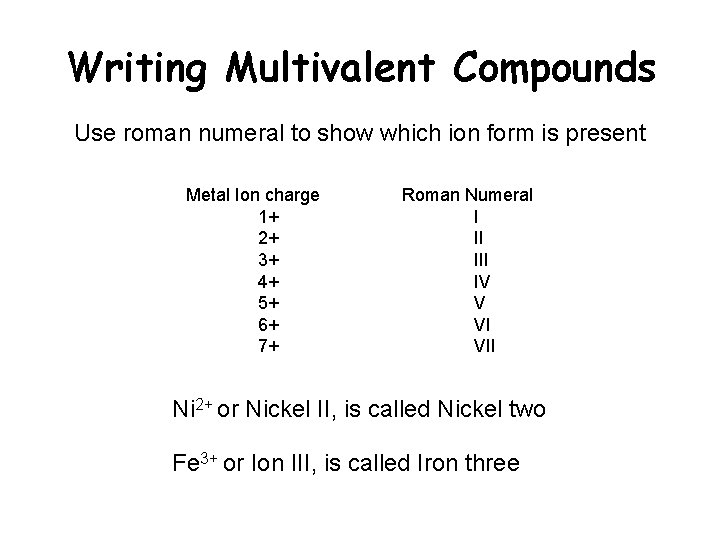
Writing Multivalent Compounds Use roman numeral to show which ion form is present Metal Ion charge 1+ 2+ 3+ 4+ 5+ 6+ 7+ Roman Numeral I II IV V VI VII Ni 2+ or Nickel II, is called Nickel two Fe 3+ or Ion III, is called Iron three

Steps for writing formulas for multivalent compounds • Step 1: Identify each ion and its charge • Step 2: Determine the total charges needed to balance positive and negative • Step 3: Note the ratio of positive ions to negative ions • Step 4: Use subscripts to write the formula, 1’s are not shown in subscript

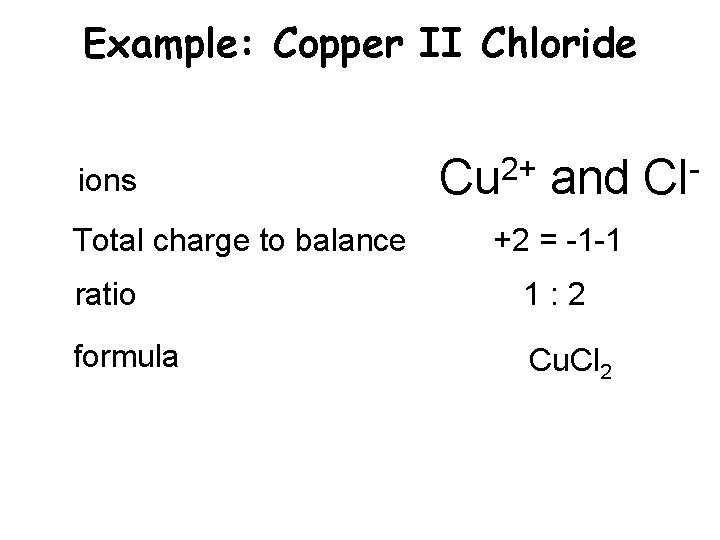
Example: Copper II Chloride ions Total charge to balance 2+ Cu and +2 = -1 -1 ratio 1: 2 formula Cu. Cl 2 Cl

Example: Manganese IV Oxide ions Total charge to balance 4+ Mn and +4 = -2 -2 ratio 1: 2 formula Mn. O 2 -2 O

Steps for naming compounds that contain a multivalent metal • Step 1: Identify the multivalent metal (first ion written in formula • Step 2: verify that it has more than one ion form • Step 3: determine the ratio of ions in the formula • Step 4: what is the charge on the negative ion? • Step 5: what must be the charge on the positive metal ion if it is to balance the negative ion • Step 6: write the name of the compound

Example: Au 3 N Au ID the metal Au+ and Au 3+ Can it be in more than 1 ion form? Au 3 N 3 Au ions for every 1 N ion Determine the ratio of ions in the formula Charge on the negative ion What does the charge on the metal have to balance the negative ion? N 3 If there are 3 Au ions then the charge on Au must be 1+ to balance out N 3 - The Name is: Gold(I)Nitride