Naming Ions chemistry 9 1 Naming Ions Naming
























- Slides: 31

Naming Ions > chemistry

9. 1 Naming Ions > Naming Ions • A rose is rosa in Spanish, warda in Arabic, and julab in Hindi. To truly understand another culture, you must first learn the language used in that culture. Similarly, to understand chemistry, you must learn its language. For this you need to know how to name ions.

9. 1 Naming Ions > Monatomic Ions • How are the charges of Group A metal and nonmetal ions related to their positions in the periodic table?

9. 1 Naming Ions > Monatomic Ions • Monatomic ions consist of a single atom with a positive or negative charge resulting from the loss or gain of one or more valence electrons, respectively.

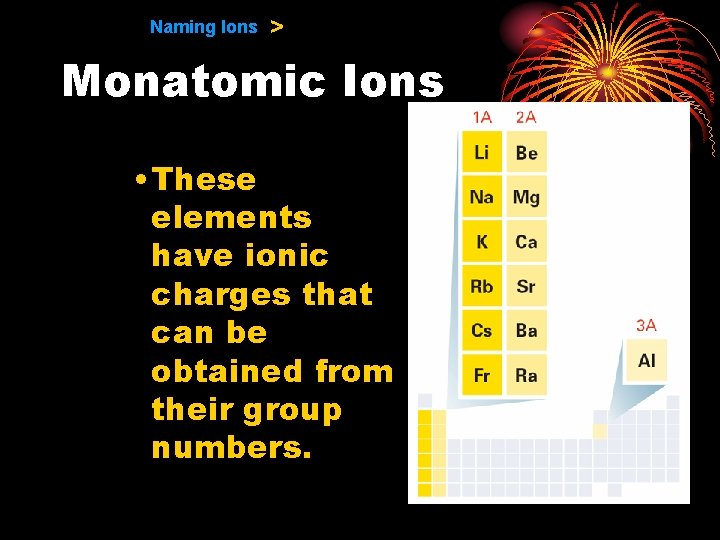
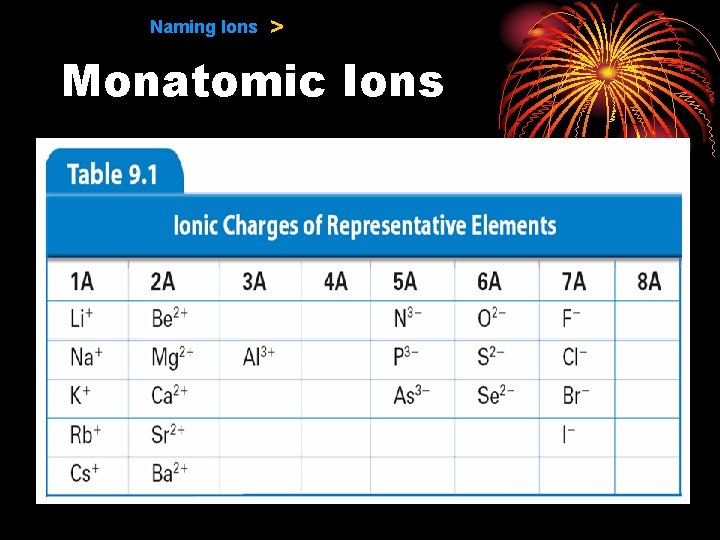
9. 1 Naming Ions > Monatomic Ions • Cations −When the metals in Groups 1 A, 2 A, and 3 A lose electrons, they form cations with positive charges equal to their group number.

9. 1 Naming Ions > Monatomic Ions • The names of the cations of the Group 1 A, Group 2 A, and Group 3 A metals are the same as the name of the metal, followed by the word ion or cation.

9. 1 Naming Ions > Monatomic Ions • These elements have ionic charges that can be obtained from their group numbers.

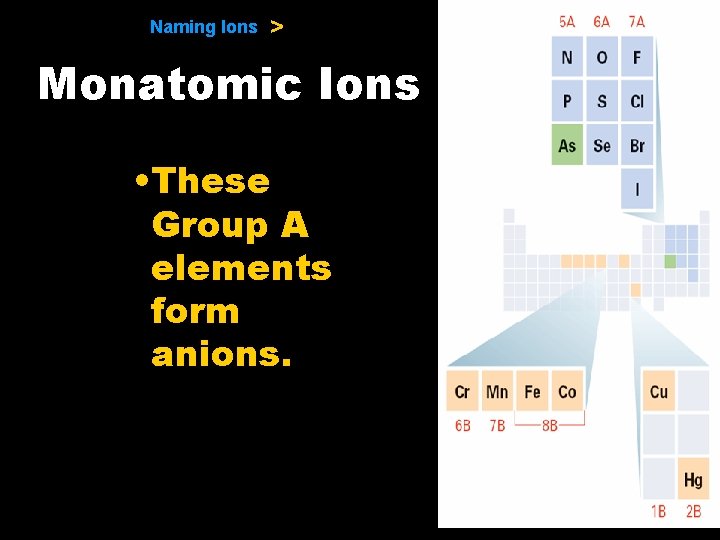
9. 1 Naming Ions > Monatomic Ions • Anions − The charge of any ion of a Group A nonmetal is determined by subtracting 8 from the group number. − Anion names start with the stem of the element name and end in -ide.

9. 1 Naming Ions > Monatomic Ions • These Group A elements form anions.

9. 1 Naming Ions > Monatomic Ions

9. 1 Naming Ions > Monatomic Ions • Ions of Transition Metals −How are the charges of some transition metal ions determined?

9. 1 Naming Ions > Monatomic Ions • The charges of the cations of many transition metal ions must be determined from the number of electrons lost.

9. 1 Naming Ions > Monatomic Ions • These colorful solutions contain the transition metal ions Co 3+, Cr 3+, Fe 3+, Ni 2+, and Mn 2+.

9. 1 Naming Ions > Monatomic Ions • Many transition metal compounds are colored and can be used as pigments.

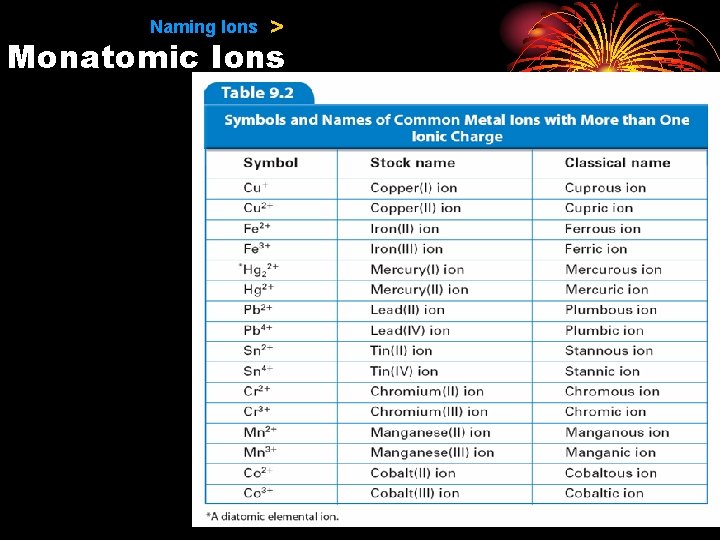
9. 1 Naming Ions > Monatomic Ions • Two methods are used to name the ions of transition metals. −The Stock system −The classical method

9. 1 Naming Ions > Monatomic Ions • In the Stock system, a Roman numeral in parentheses is placed after the name of the element to indicate the numerical value of the charge.

9. 1 Naming Ions > Monatomic Ions • In an older, less useful method, the classical name of the element is used to form the root name for the element.

9. 1 Naming Ions > Monatomic Ions

Naming Ions 1. 1 > Conceptual Problem 9. 1

Naming Ions 1. 1 > Conceptual Problem 9. 1

Naming Ions 1. 1 > Conceptual Problem 9. 1

9. 1 Naming Ions > Polyatomic Ions • Polyatomic Ions • What are the two endings of the names of most polyatomic ions?

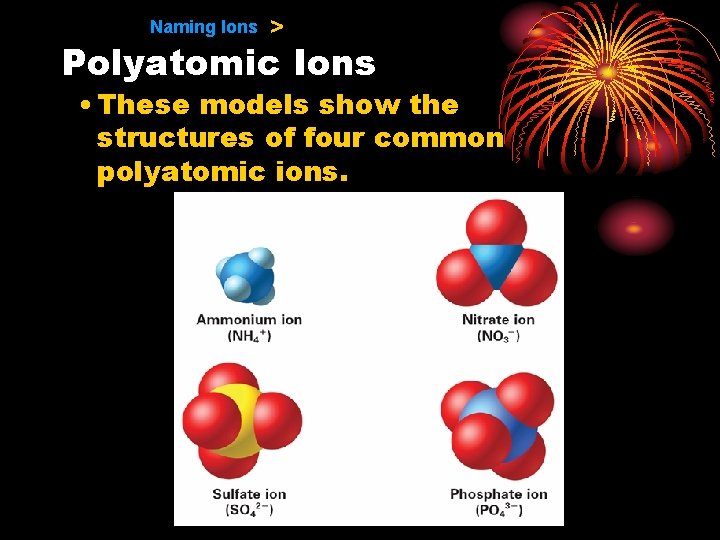
9. 1 Naming Ions > Polyatomic Ions • These models show the structures of four common polyatomic ions.

9. 1 Naming Ions > Polyatomic Ions • Some ions, called polyatomic ions, are composed of more than one atom. − The names of most polyatomic anions end in -ite or -ate.

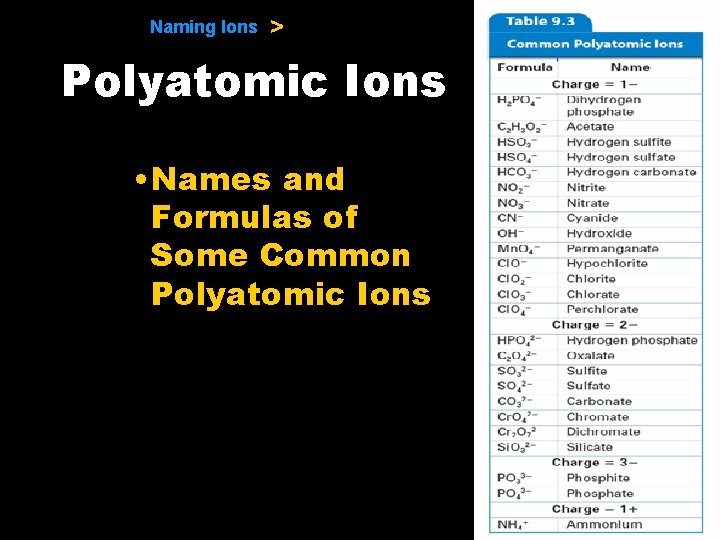
9. 1 Naming Ions > Polyatomic Ions • Names and Formulas of Some Common Polyatomic Ions

9. 1 Naming Ions > Polyatomic Ions • Sodium hydrogen carbonate can relieve an upset stomach.

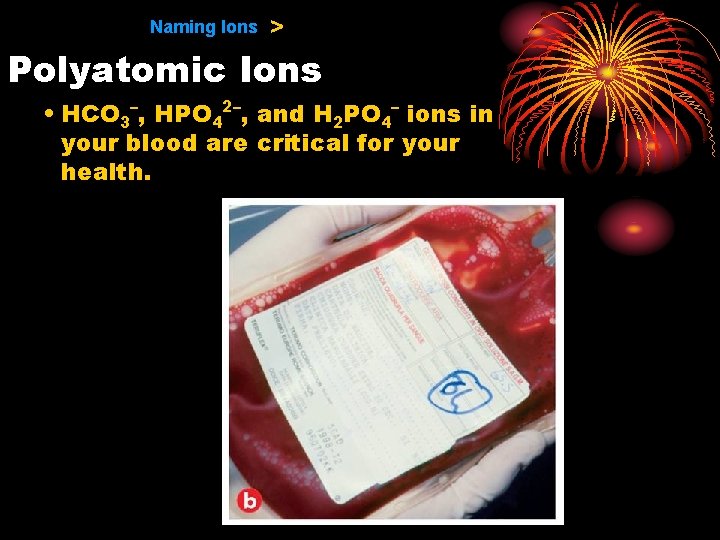
9. 1 Naming Ions > Polyatomic Ions • HCO 3–, HPO 42–, and H 2 PO 4– ions in your blood are critical for your health.

9. 1 Naming Ions > Polyatomic Ions • Fertilizers contain HPO 42– and H 2 PO 4– ions.

Naming Ions > 9. 1 Section Quiz. • 9. 1.

Naming Ions > 9. 1 Section Quiz. • 1. When metals from groups 1 A, 2 A, and 3 A form cations, the charge on the ion is equal to a. the group number minus 8. b. 8 minus the group number. c. the period number. d. the group number.

Naming Ions > END OF SHOW