Naming Ionic Compounds with Polyatomic Ions Complex Ionic






- Slides: 12

Naming Ionic Compounds with Polyatomic Ions Complex Ionic Compounds

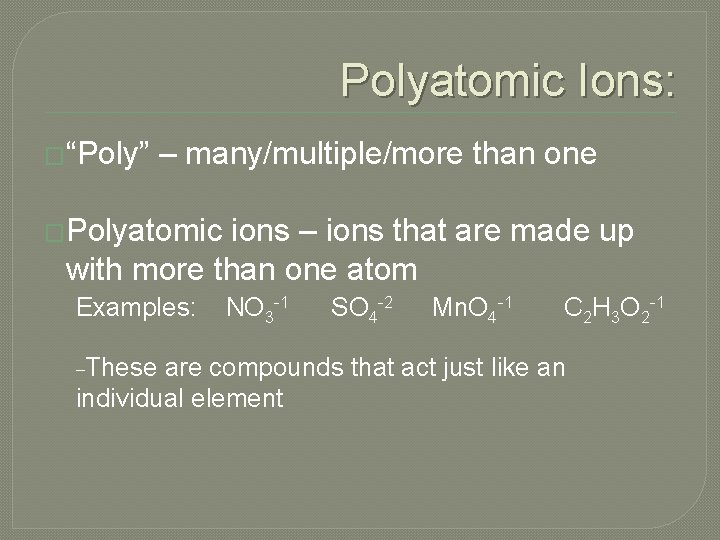
Polyatomic Ions: �“Poly” – many/multiple/more than one �Polyatomic ions – ions that are made up with more than one atom Examples: _These NO 3 -1 SO 4 -2 Mn. O 4 -1 C 2 H 3 O 2 -1 are compounds that act just like an individual element

Complex Ionic Compounds �Any ionic compound that contains three or more elements. �It can ONLY occur if there is a polyatomic ion present in the compound.

This is your list, please do not lose it

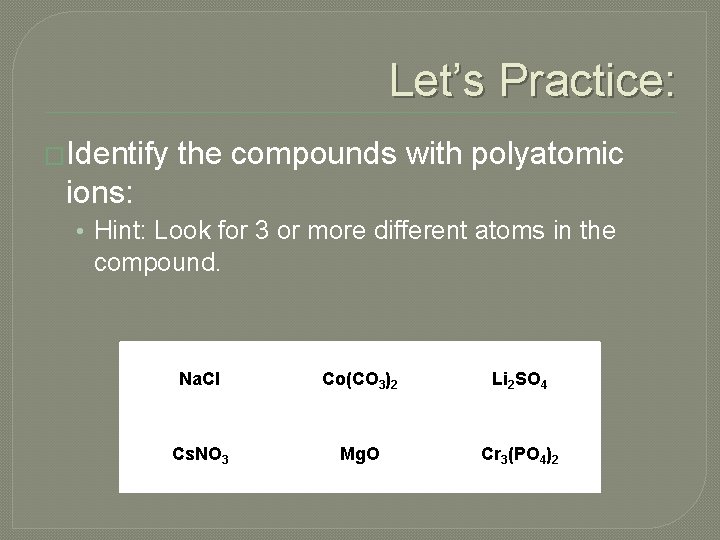
Let’s Practice: �Identify the compounds with polyatomic ions: • Hint: Look for 3 or more different atoms in the compound. Na. Cl Co(CO 3)2 Li 2 SO 4 Cs. NO 3 Mg. O Cr 3(PO 4)2

Rules for naming Complex Ionic Compounds: 1. Name the first element directly off the periodic table, IF IT IS A METAL *Remember to identify the charge of any transition metal using roman numerals 2. Name the second element, IF IT IS A NONMETAL, by changing the ending to –ide 3. If either of the elements is a polyatomic, name it directly off the polyatomic list, replacing step 1 or 2. 4. Always double check the oxidation number of the anion, polyatomic or single ion, if the cation is a transition metal. Na 2 CO 3 Sodium Carbonate

Naming Complex Ionic Compounds: 1. Name the first element directly off the periodic table, IF IT IS A METAL *Remember to identify the charge of any transition metal using roman numerals 2. Name the second element, IF IT IS A NONMETAL, by changing the ending to –ide 3. If either of the elements is a polyatomic, name it directly off the polyatomic list, replacing step 1 or 2. 4. Always double check the oxidation number of the anion, polyatomic or single ion, if the cation is a transition metal. NH 4 Cl Ammonium Chloride

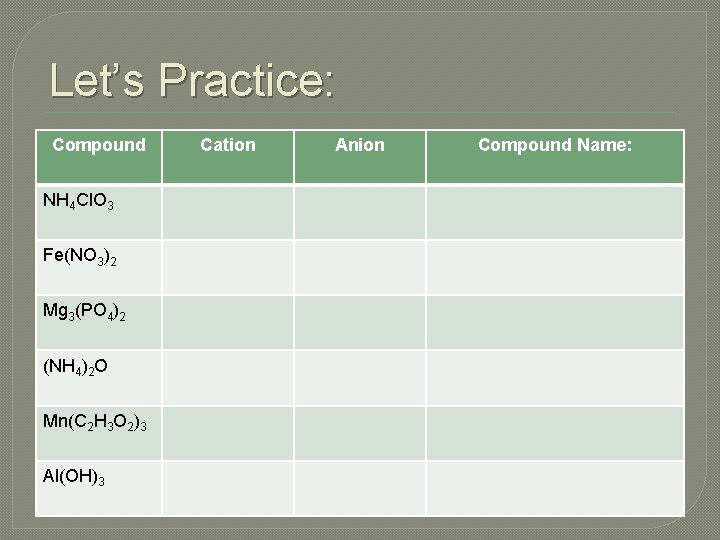
Let’s Practice: Compound NH 4 Cl. O 3 Fe(NO 3)2 Mg 3(PO 4)2 (NH 4)2 O Mn(C 2 H 3 O 2)3 Al(OH)3 Cation Anion Compound Name:

Rules for writing compound formulas: 1. 2. 3. 4. 5. 6. Write out the ions for each of the elements/polyatomic present Write the charge with each ion present Drop the signs (+/-) Swap the numbers Simplify if necessary Cage the Beast – put the polyatomic ion(s) in parenthesis no matter how many you need. Sodium Phosphate Na+1 PO 4 -3 Na 3(PO 4)

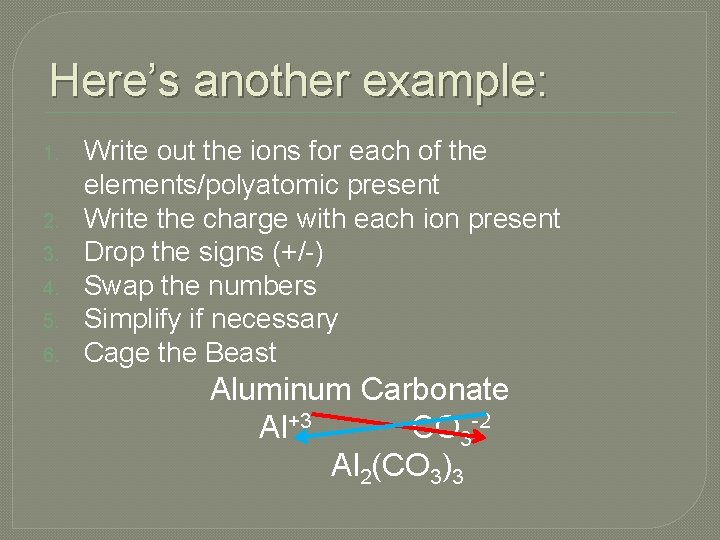
Here’s another example: 1. 2. 3. 4. 5. 6. Write out the ions for each of the elements/polyatomic present Write the charge with each ion present Drop the signs (+/-) Swap the numbers Simplify if necessary Cage the Beast Aluminum Carbonate Al+3 CO 3 -2 Al 2(CO 3)3

Writing formulas for complex ionic compounds: � When we need multiple polyatomic ions to create a neutral compound, ( ) are used, like always, around the polyatomic ion and its subscript. � The new subscript (from the swap and drop) is placed OUTSIDE of the ( ) � The polyatomic ion MUST be treated as ONE atom…. it is a unit that CANNOT be separated. Ca+2 Cl. O 3 -1 Ca(Cl. O 3)2 Calcium Chlorate

Let’s Practice: Compound Name Beryllium Nitrate Chromium (II) Hydroxide Gallium Chlorate Ammonium Carbonate Zirconium (III) Sulfate Cation symbol Anion symbol Compound