Naming Ionic Compounds Science 10 Simple Ionic Compounds
















- Slides: 19

Naming Ionic Compounds Science 10

Simple Ionic Compounds Ø The rules: l Name the first element • This is always the metal (cation) • The name stays as it appears on your periodic table l Name the second element • This is always the non-metal (anion) • The name will be similar to that on the periodic table with the ending –ide

Ø The two element names are put together with a space in the middle Ø We ignore any numbers in the name – they are only there to make the charges equal

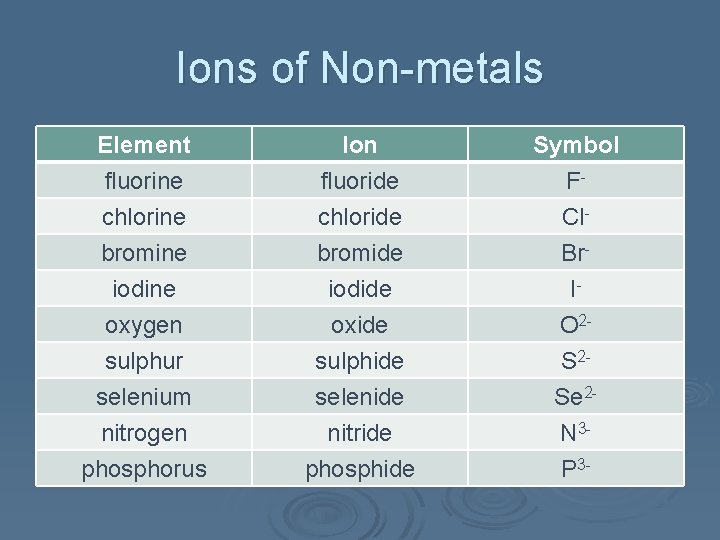
Ions of Non-metals Element fluorine chlorine bromine Ion fluoride chloride bromide Symbol FCl. Br- iodine oxygen sulphur selenium nitrogen phosphorus iodide oxide sulphide selenide nitride phosphide IO 2 S 2 Se 2 N 3 P 3 -

Example Ø KBr l l l First = potassium Second = bromide Name: potassium bromide Ø Ca. F 2 l l l First = calcium Second = fluoride Name: calcium fluoride

You Try 1. Mg. Cl 2 2. Ca. O 3. Sc 2 O 3 4. Na 2 O

Ionic Compound with Multivalent Elements Ø Some elements are able to form more than one ion – they are called multivalent Ø Example: Copper can be Cu+ or Cu 2+ meaning it can donate 1 or 2 electrons Ø On your periodic table of ions, both charges will be shown

When compounds are made with multivalent elements, we must indicate which charge is used. Ø This is done by writing the roman numeral of the charge next to the name of the cation Ø Ø Example: Fe. O 2+ is required to balance with O 2 l Fe l The name is: iron(II) oxide

You Try 1. Mn. Cl 2 2. Ni 2 S 3 3. Cu. O 4. Cu. Br 2

Writing a Chemical Formula If you’re given the name, can you write the formula? Ø Rules: l First: find the ion for the first element l Second: find the ion for the second element l Third: use the criss-cross rule to make the formula

Three Line Method Ø The three line method helps us do this l l l Line one is the name Line two are the ions Line three is the chemical formula Line one = name Line two = ions Line three = chemical formula

Example Ø strontium nitride Ø sodium oxide

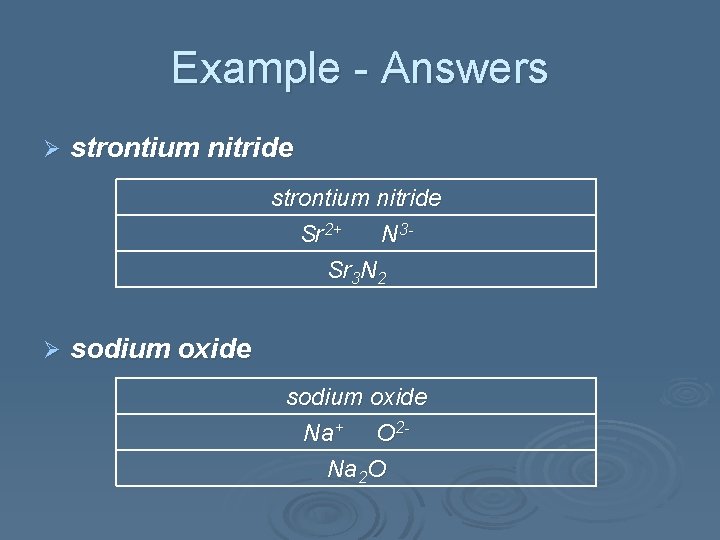
Example - Answers Ø strontium nitride Sr 2+ N 3 Sr 3 N 2 Ø sodium oxide Na+ O 2 Na 2 O

You Try Write the formula’s for: Ø calcium oxide Ø potassium sulphide Ø zinc chloride Ø copper(I) phosphide

Naming Ionic Compounds With Polyatomic Ions

Polyatomic Ions Ø Polyatomic ions are ions that contain two or more atoms. Ø They have a charge which means that they will gain and lose electrons Ø This allows them to be part of an ionic compound Ø A list of polyatomic ions is found on the back of your periodic table

Naming with Polyatomic Ions Ø It is the same for naming ionic compounds BUT you must use the names of the polyatomic ions. Ø The endings will not change to –ide Ø Examples: l Li. OH l NH 4 Cl l Ba. SO 3

Writing Formula’s Ø When there is more than one of the polyatomic ion needed you must place it in brackets Ø Example: lithium hydroxide Ø Example: calcium hydroxide

You Try Ø Write the name or formula for the following: 1. 2. 3. 4. 5. sodium hydroxide Sr(NO 2) magnesium nitrate Na. PO 4 calcium carbonate