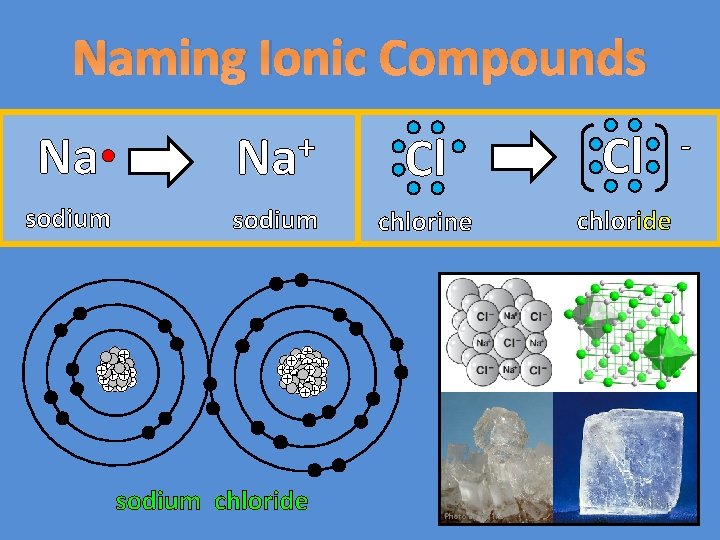
Naming Ionic Compounds Na Na Cl Cl sodium







- Slides: 25

Naming Ionic Compounds Na + Na Cl Cl sodium chlorine chloride sodium chloride Photo by Ra’ike -

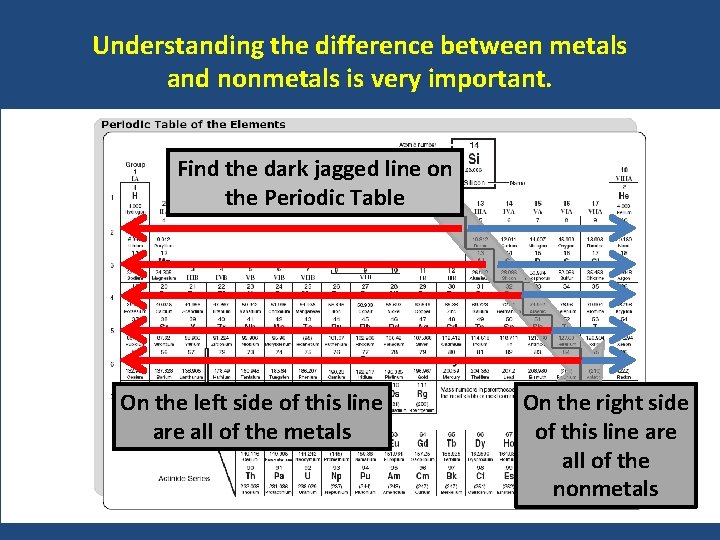
Understanding the difference between metals and nonmetals is very important. Find the dark jagged line on the Periodic Table On the left side of this line are all of the metals On the right side of this line are all of the nonmetals

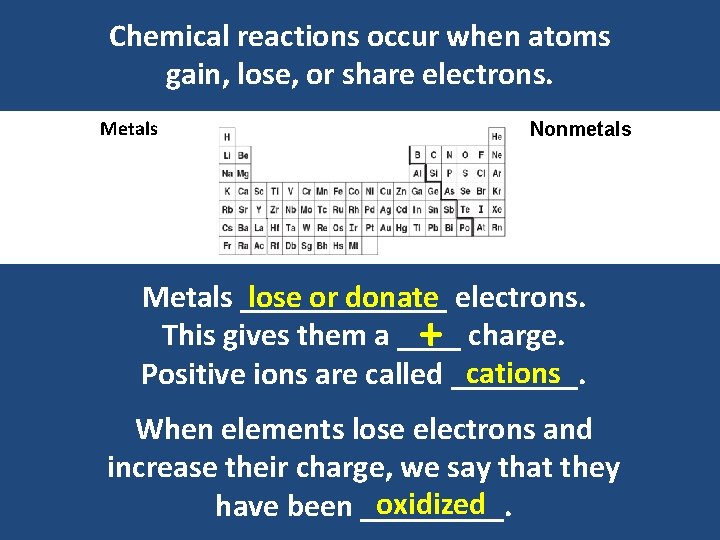
Chemical reactions occur when atoms gain, lose, or share electrons. Metals Nonmetals Metals _______ lose or donate electrons. This gives them a ____ + charge. cations Positive ions are called ____. When elements lose electrons and increase their charge, we say that they oxidized have been _____.

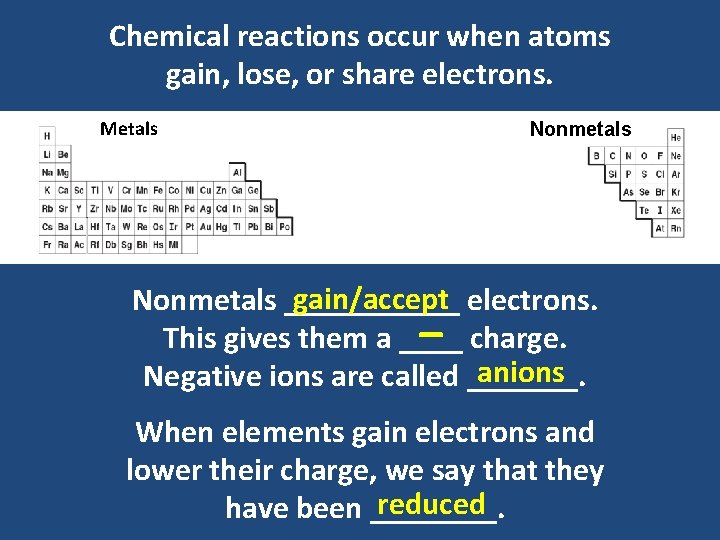
Chemical reactions occur when atoms gain, lose, or share electrons. Metals Nonmetals gain/accept electrons. Nonmetals ______ This gives them a ____ charge. anions Negative ions are called _______. _ When elements gain electrons and lower their charge, we say that they reduced have been ____.

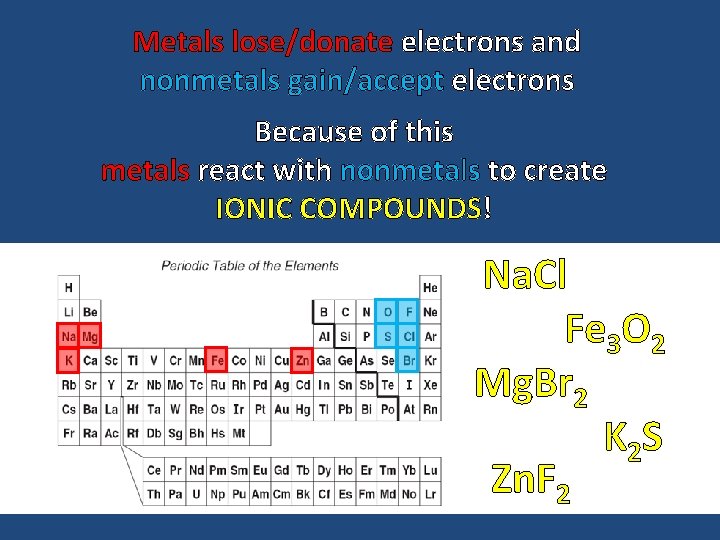
Metals lose/donate electrons and nonmetals gain/accept electrons Because of this metals react with nonmetals to create IONIC COMPOUNDS! Na. Cl Fe 3 O 2 Mg. Br 2 K 2 S Zn. F 2

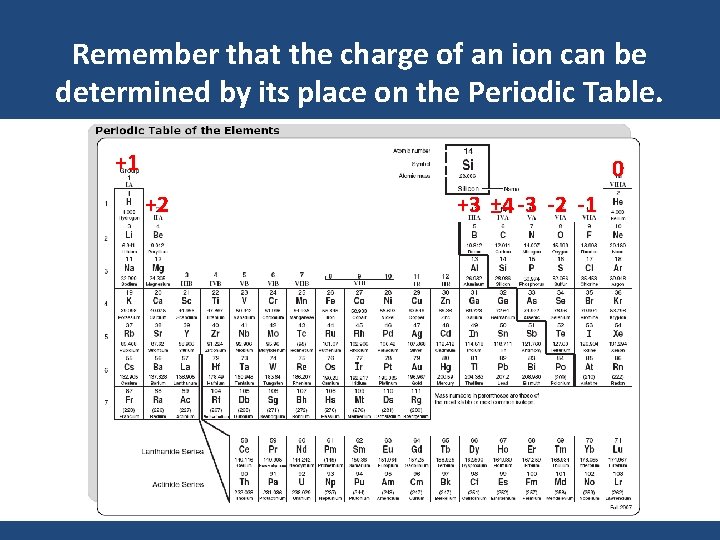
Remember that the charge of an ion can be determined by its place on the Periodic Table. +1 0 +2 +3 ± 4 -3 -2 -1

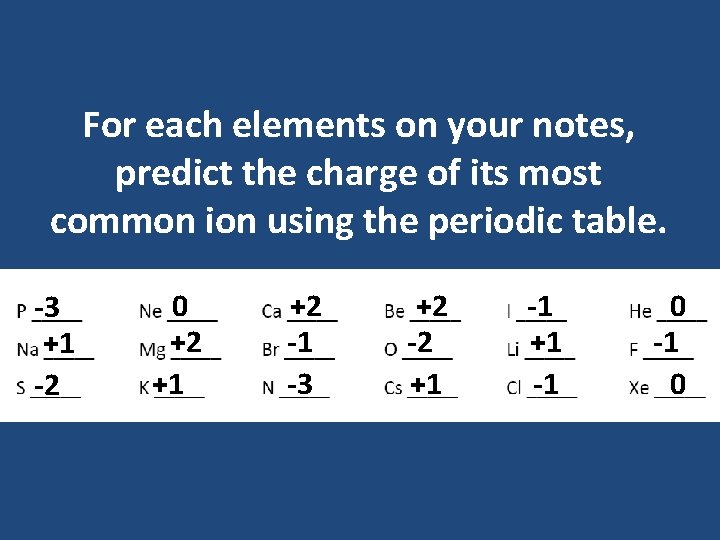
For each elements on your notes, predict the charge of its most common ion using the periodic table. -3 +1 -2 0 +2 +1 +2 -1 -3 +2 -2 +1 -1 0

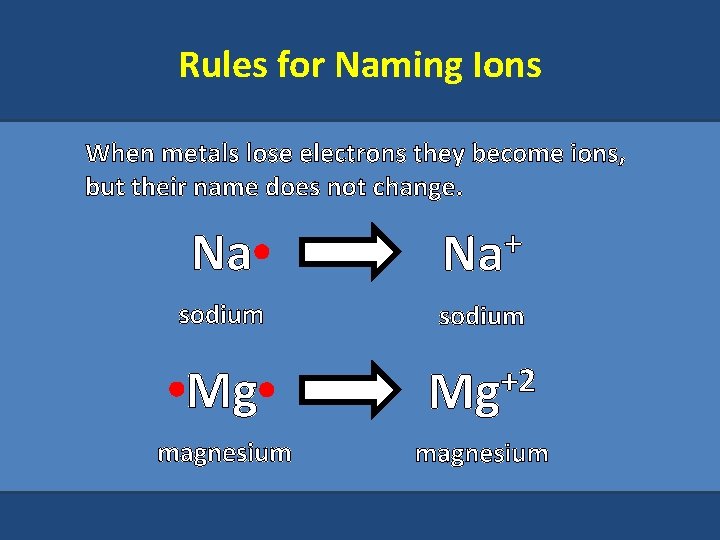
Rules for Naming Ions When metals lose electrons they become ions, but their name does not change. Na + Na sodium Mg +2 Mg magnesium

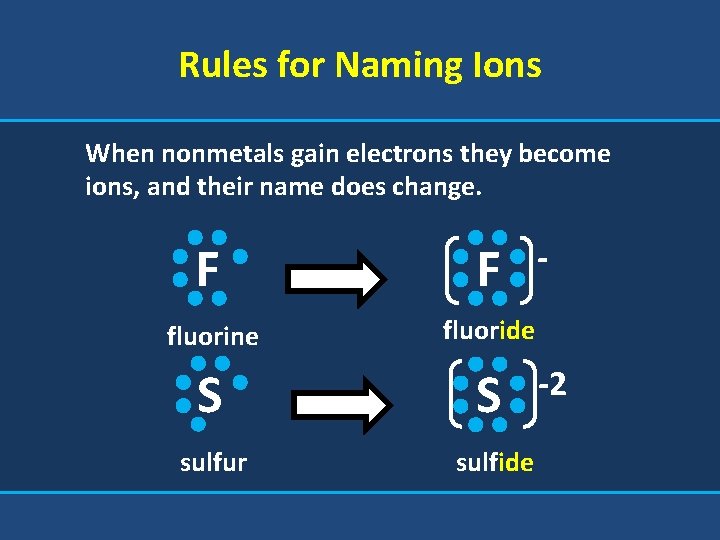
Rules for Naming Ions When nonmetals gain electrons they become ions, and their name does change. F F fluorine fluoride S S sulfur sulfide -2

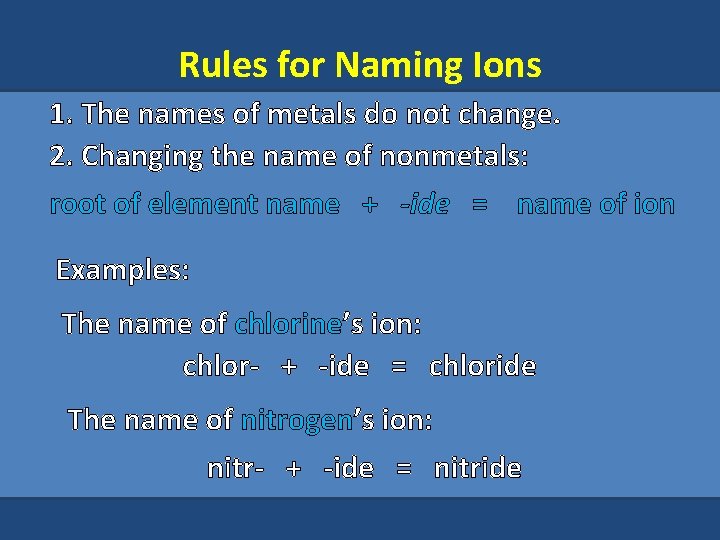
Rules for Naming Ions 1. The names of metals do not change. 2. Changing the name of nonmetals: root of element name + -ide = name of ion Examples: The name of chlorine’s ion: chlor- + -ide = chloride The name of nitrogen’s ion: nitr- + -ide = nitride

Examples of naming ions: The name of calcium’s ion: calcium (The names of metals don’t change!) The name of oxygen’s ion: ox- + -ide = oxide The name of aluminum’s ion: aluminum (The names of metals don’t change!)

Write the name of each of the ions on your notes. sulfide nitride potassium oxide lithium bromide chloride hydrogen (+), hydride (-)

It is also important that you can identify the names of polyatomic ions. Polyatomic ions are covalently bonded elements that have a charge!

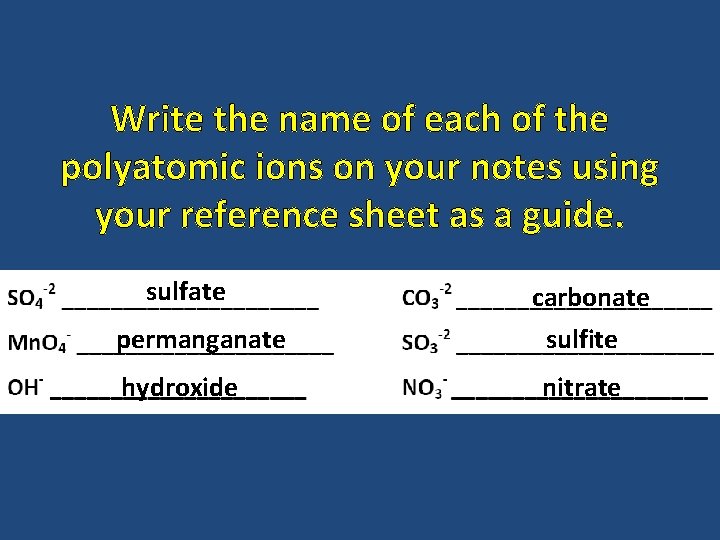
Write the name of each of the polyatomic ions on your notes using your reference sheet as a guide. sulfate permanganate hydroxide carbonate sulfite nitrate

Steps for Naming Ionic Compounds Ca. Br 2 calcium bromide Step 1: Write the name of the metal ion. Step 2: Write the name of the nonmetal ion. YOU ARE DONE! It is that easy.

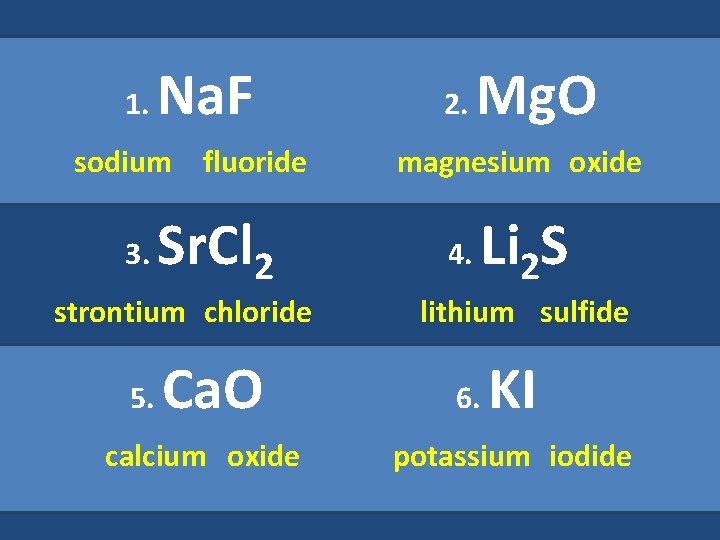
1. Na. F sodium fluoride 3. Sr. Cl 2 strontium chloride 5. Ca. O calcium oxide 2. Mg. O magnesium oxide 4. Li 2 S lithium sulfide 6. KI potassium iodide

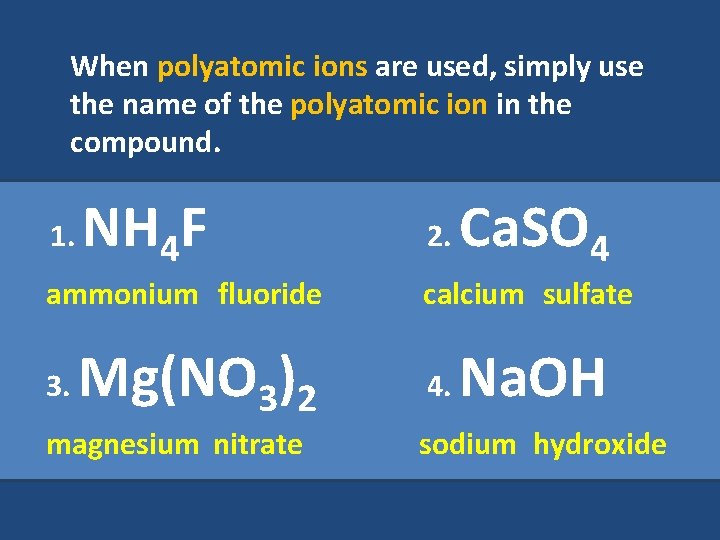
When polyatomic ions are used, simply use the name of the polyatomic ion in the compound. 1. NH 4 F ammonium fluoride 3. Mg(NO 3)2 magnesium nitrate 2. Ca. SO 4 calcium sulfate 4. Na. OH sodium hydroxide

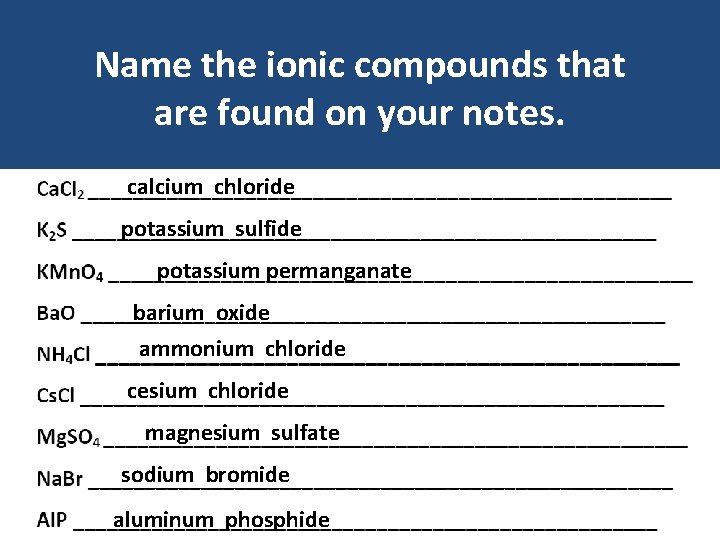
Name the ionic compounds that are found on your notes. calcium chloride potassium sulfide potassium permanganate barium oxide ammonium chloride cesium chloride magnesium sulfate sodium bromide aluminum phosphide

You can also determine the formula of an ionic compound from its name. To do this, you will need to use what you already know about the Periodic Table. magnesium iodide +2 Mg I Mg. I 2 - Step 1: Write the symbol and charge of the metal ion using the Periodic Table. Step 2: Write the symbol and charge of the nonmetal ion using the Periodic Table. Step 3: Determine the formula from the ions.

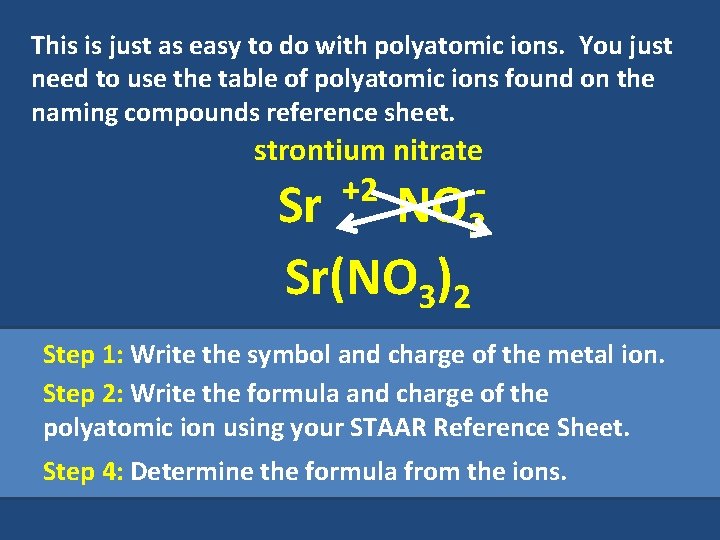
This is just as easy to do with polyatomic ions. You just need to use the table of polyatomic ions found on the naming compounds reference sheet. strontium nitrate +2 NO 3 Sr Sr(NO 3)2 Step 1: Write the symbol and charge of the metal ion. Step 2: Write the formula and charge of the polyatomic ion using your STAAR Reference Sheet. Step 4: Determine the formula from the ions.

Be very careful that you do not mix up the names of ions. This is very common for beginners to naming. Decide which name goes with each ion. -3 N nitrate nitride NO 3 -2 S sulfide sulfite SO 3 phosphate -3 P phosphide PO 4 - -2 -3

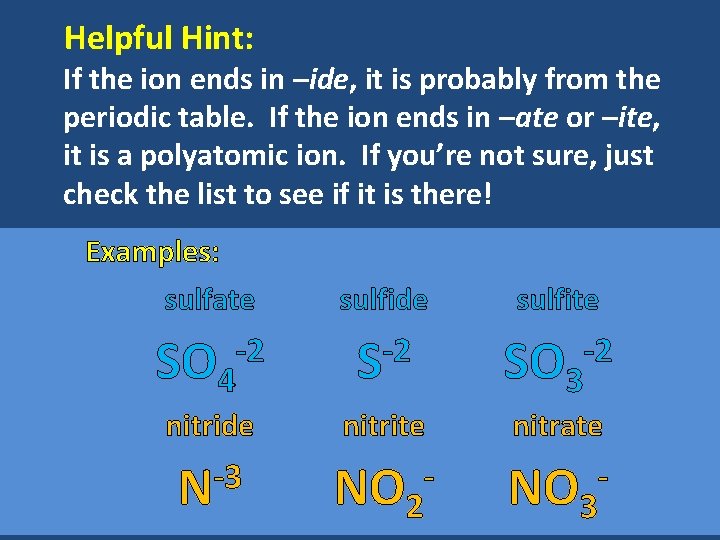
Helpful Hint: If the ion ends in –ide, it is probably from the periodic table. If the ion ends in –ate or –ite, it is a polyatomic ion. If you’re not sure, just check the list to see if it is there! Examples: sulfate SO 4 -2 sulfide -2 S sulfite SO 3 -2 nitride nitrite nitrate -3 N NO 2 NO 3 - -

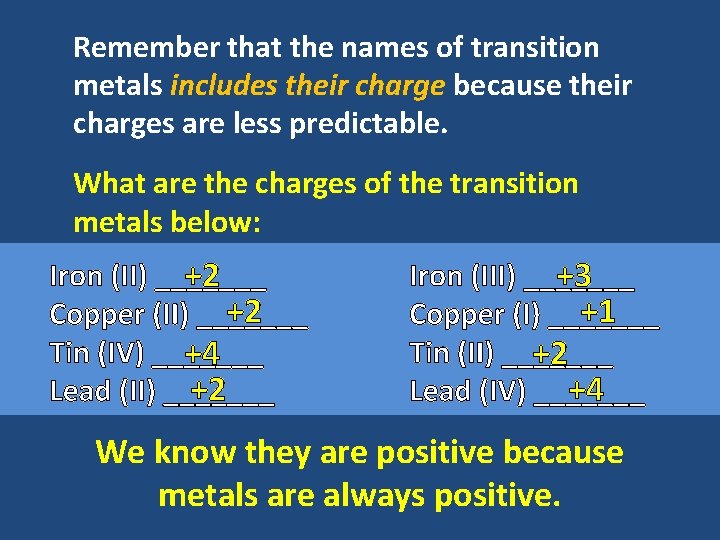
Remember that the names of transition metals includes their charge because their charges are less predictable. What are the charges of the transition metals below: Iron (II) _______ +2 +2 Copper (II) _______ Tin (IV) _______ +4 +2 Lead (II) _______ Iron (III) _______ +3 +1 Copper (I) _______ Tin (II) _______ +2 +4 Lead (IV) _______ We know they are positive because metals are always positive.

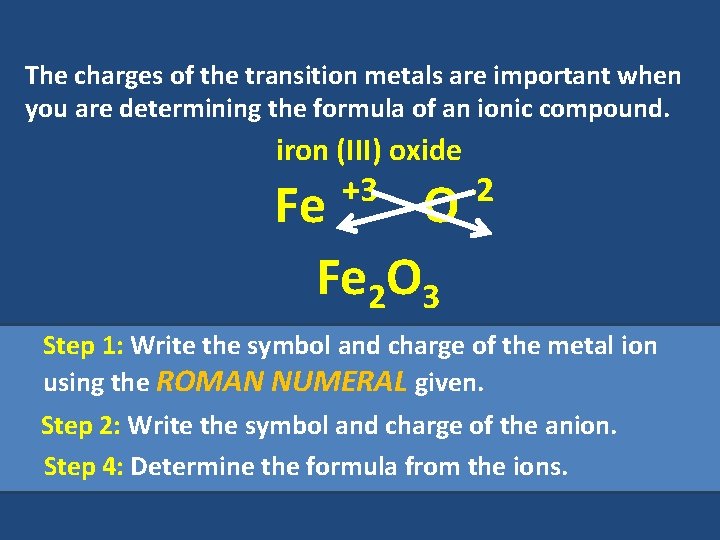
The charges of the transition metals are important when you are determining the formula of an ionic compound. iron (III) oxide +3 Fe O Fe 2 O 3 -2 Step 1: Write the symbol and charge of the metal ion using the ROMAN NUMERAL given. Step 2: Write the symbol and charge of the anion. Step 4: Determine the formula from the ions.

Write the formula of each of the ionic compounds named on your notes. KI Sn. Cl 4 Ba. SO 4 Na. Cl Sr. S Cu. CO 3 Al. Br 3 Li 3 N