Naming Ionic Compounds Binary Compounds Rules for Naming

Naming Ionic Compounds Binary Compounds

Rules for Naming Ionic Compounds • For the Cation • Borrow the name of the element • Ex. K+ is the “Potassium Ion” • Ex. Zn 2+ is the “Zinc Ion” • If an ion has more than one oxidation state • Cu+1 is “copper one ion” • Cu+2 is “copper two ion”

Rules for naming the anion • Anion- the negatively charged atom • Use the name of the element followed by the suffix “-ide” • Cl- is the Chloride ion • O-2 is the Oxide ion • P-3 is the Phosphide ion

Naming Ionic Compounds • When naming ionic binary compounds: • Name the cation’s element name followed by the anion’s name • • • Na. Cl is “Sodium Chloride” K 2 O is “Potassium Oxide” Cu. Cl 2 is “Copper (II) Chloride” Mg 3 N 2 is “Magnesium Nitride” Al 2 S 3 is “Aluminum Sulfide”
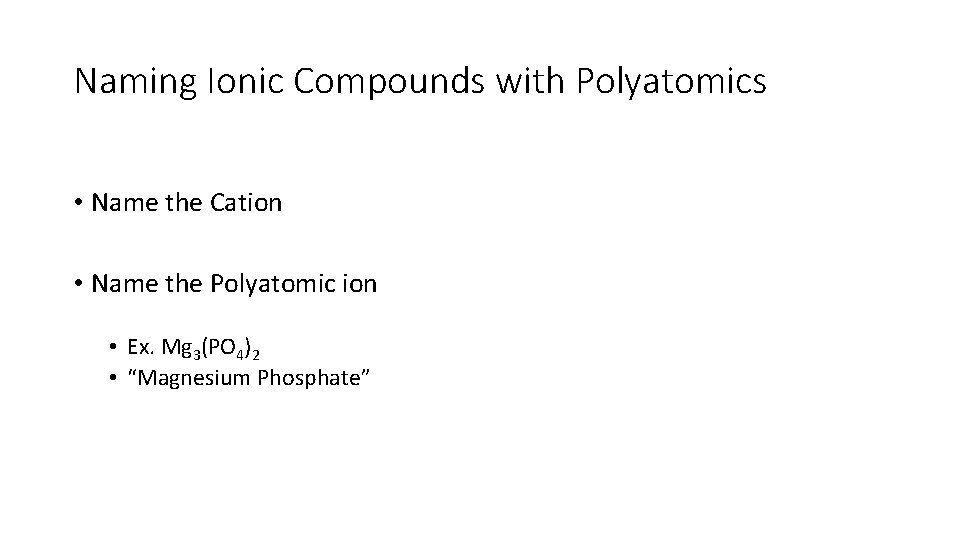
Naming Ionic Compounds with Polyatomics • Name the Cation • Name the Polyatomic ion • Ex. Mg 3(PO 4)2 • “Magnesium Phosphate”

Name this compound: Fe. O X Iron oxide Fe+2 O-2 Iron (II) oxide If that’s correct, name this: Fe 2 O 3 Fe+3 O-2 Iron (III) oxide How can we distinguish between these compounds? What is the charge on the oxygen in each compound? Then what is the charge on the iron in each compound? Fix the name of the first compound:
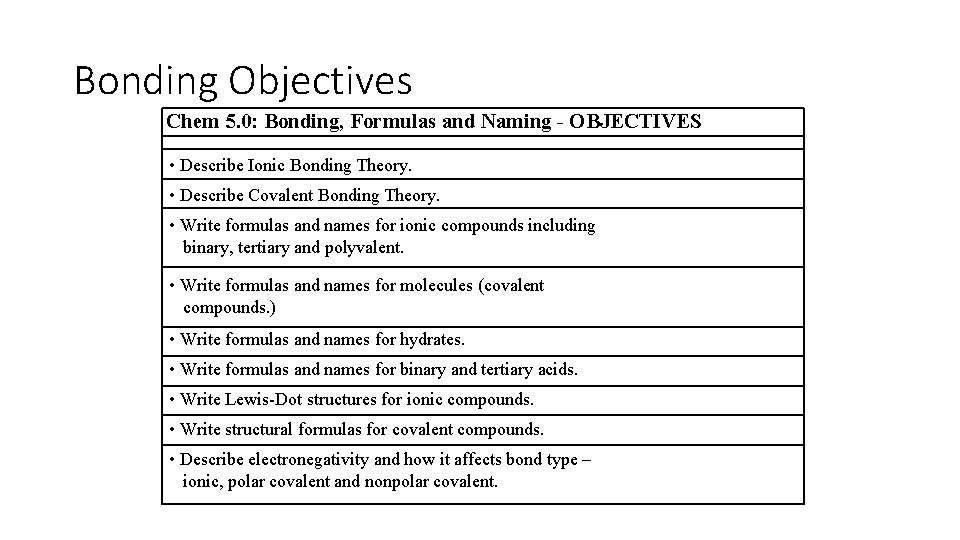
Bonding Objectives Chem 5. 0: Bonding, Formulas and Naming - OBJECTIVES • Describe Ionic Bonding Theory. • Describe Covalent Bonding Theory. • Write formulas and names for ionic compounds including binary, tertiary and polyvalent. • Write formulas and names for molecules (covalent compounds. ) • Write formulas and names for hydrates. • Write formulas and names for binary and tertiary acids. • Write Lewis-Dot structures for ionic compounds. • Write structural formulas for covalent compounds. • Describe electronegativity and how it affects bond type – ionic, polar covalent and nonpolar covalent.
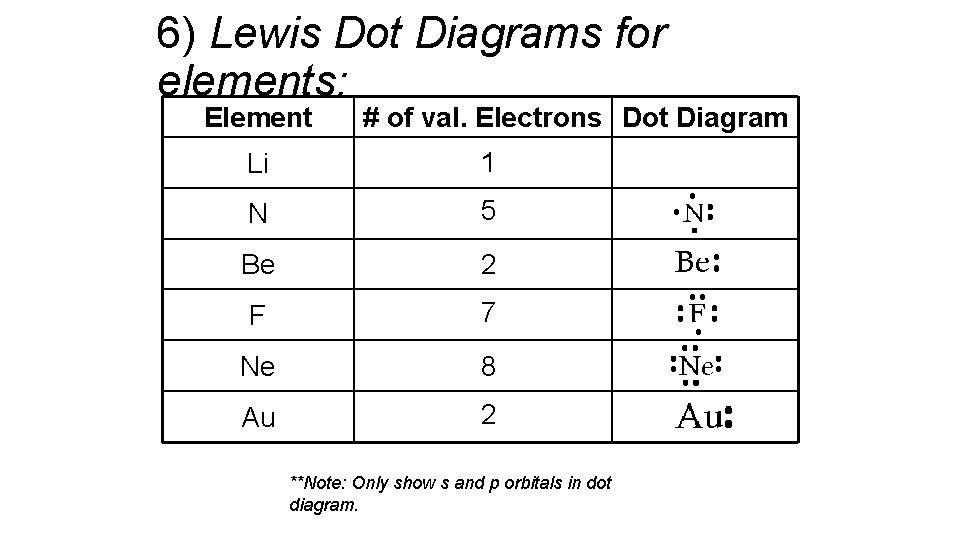
6) Lewis Dot Diagrams for elements: Element # of val. Electrons Dot Diagram Li 1 N 5 Be 2 F 7 Ne 8 Au 2 **Note: Only show s and p orbitals in dot diagram. Li
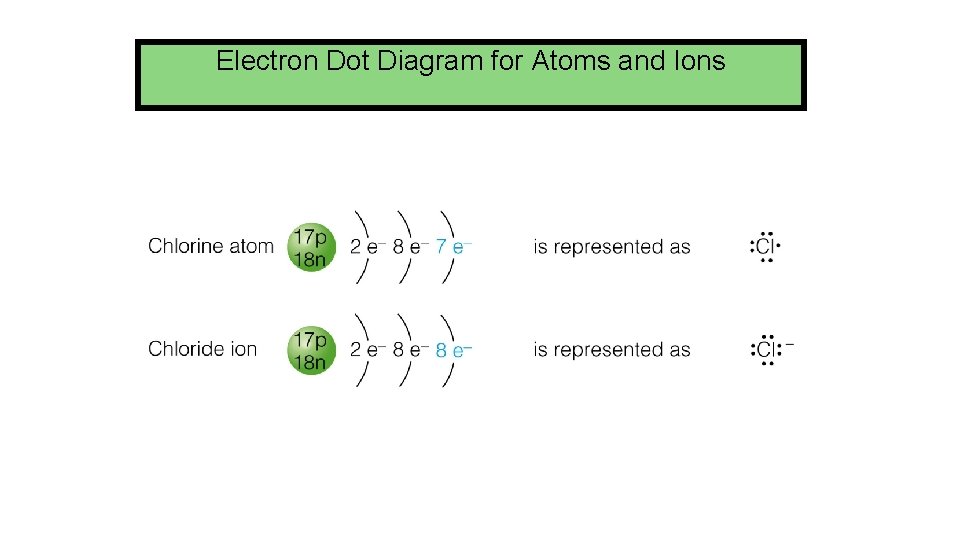
Electron Dot Diagram for Atoms and Ions
![7) Lewis-dot diagram for sodium chloride: [Na+] [ -] 7) Lewis-dot diagram for sodium chloride: [Na+] [ -]](http://slidetodoc.com/presentation_image_h2/8581946b2c3856f31349630d58ab6bd6/image-10.jpg)
7) Lewis-dot diagram for sodium chloride: [Na+] [ -]
![Lewis-dot diagram for Na. Cl Na Ionic Bond Formed + Na [Na+] [ -] Lewis-dot diagram for Na. Cl Na Ionic Bond Formed + Na [Na+] [ -]](http://slidetodoc.com/presentation_image_h2/8581946b2c3856f31349630d58ab6bd6/image-11.jpg)
Lewis-dot diagram for Na. Cl Na Ionic Bond Formed + Na [Na+] [ -] Na
Bonding Objectives Chem 5. 0: Bonding, Formulas and Naming - OBJECTIVES • Describe Ionic Bonding Theory. • Describe Covalent Bonding Theory. • Write formulas and names for ionic compounds including binary, tertiary and polyvalent. • Write formulas and names for molecules (covalent compounds. ) • Write formulas and names for hydrates. • Write formulas and names for binary and tertiary acids. • Write Lewis-Dot structures for ionic compounds. • Write structural formulas for covalent compounds. • Describe electronegativity and how it affects bond type – ionic, polar covalent and nonpolar covalent.
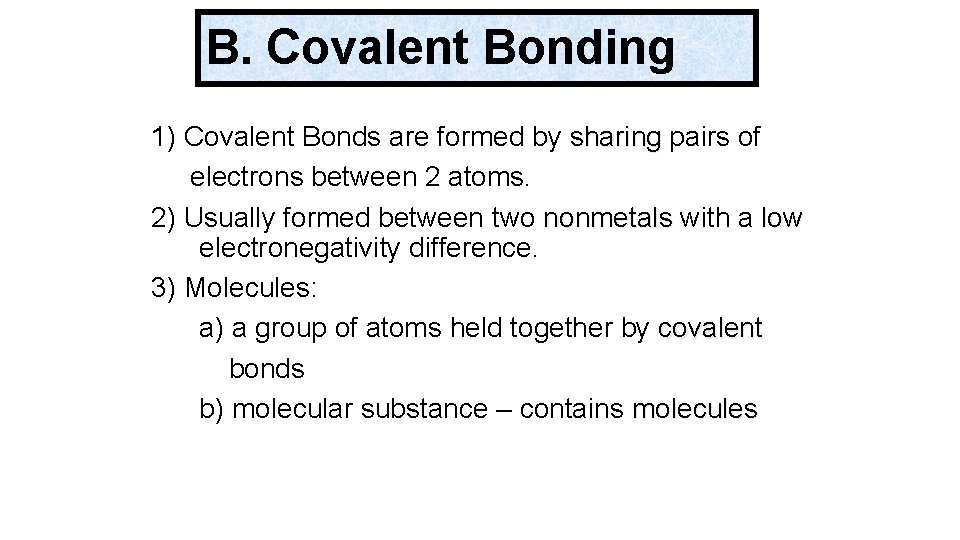
B. Covalent Bonding 1) Covalent Bonds are formed by sharing pairs of electrons between 2 atoms. 2) Usually formed between two nonmetals with a low electronegativity difference. 3) Molecules: a) a group of atoms held together by covalent bonds b) molecular substance – contains molecules

4) Naming: a) uses prefixes: 1 mono- 4 tetradeca- 2 di 3 tri- 7 hepta- 10 5 penta- 8 octa 6 hexa- 9 nona- b) end in “ide” c) More electronegative element is written last d) Only use a prefix on the first element if it is more than one e) Always use a prefix for the second element Ex) water H 2 O dihydrogen monoxide smog NO 2 nitrogen dioxide

SF 6 (Sulfur Hexafluoride) Cool little video
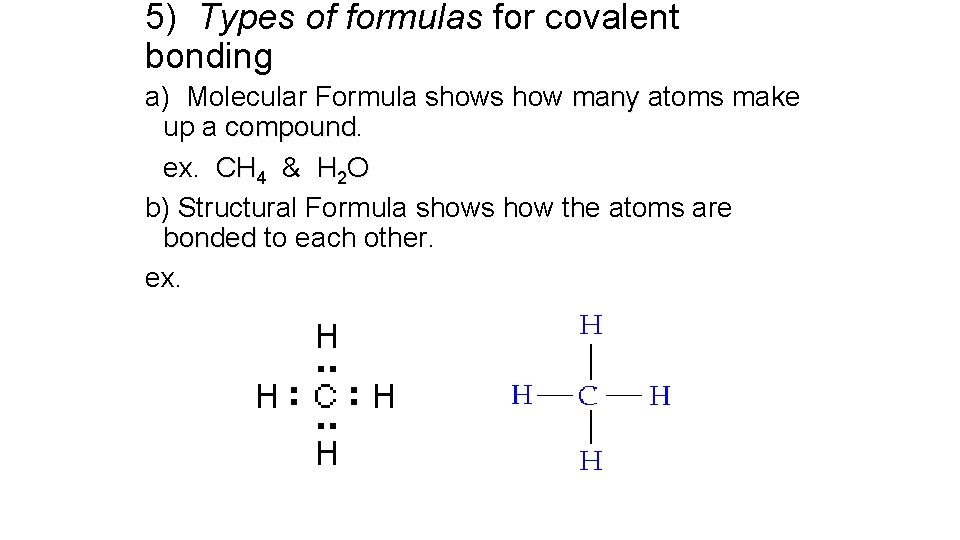
5) Types of formulas for covalent bonding a) Molecular Formula shows how many atoms make up a compound ex. CH 4 & H 2 O b) Structural Formula shows how the atoms are bonded to each other. ex.
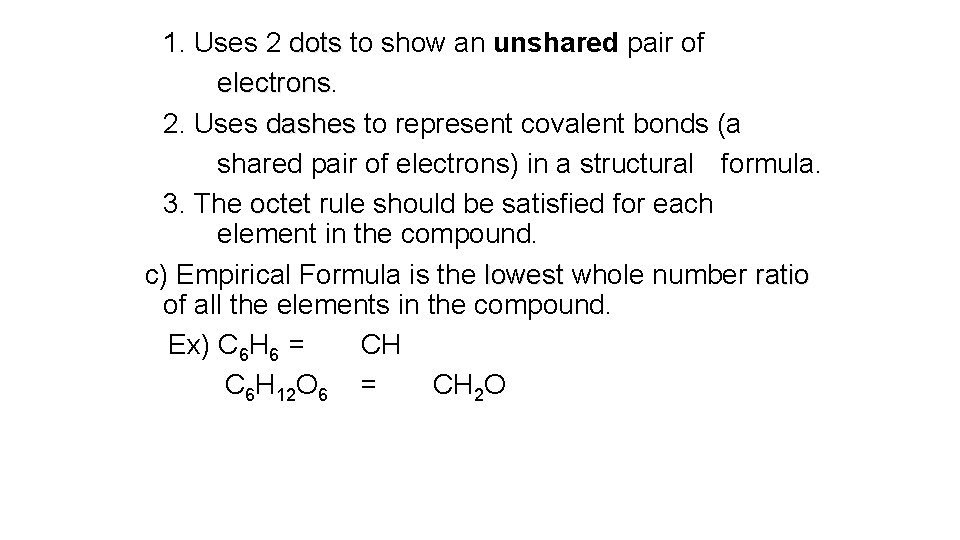
1. Uses 2 dots to show an unshared pair of electrons 2. Uses dashes to represent covalent bonds (a shared pair of electrons) in a structural formula. 3. The octet rule should be satisfied for each element in the compound. c) Empirical Formula is the lowest whole number ratio of all the elements in the compound. Ex) C 6 H 6 = CH C 6 H 12 O 6 = CH 2 O
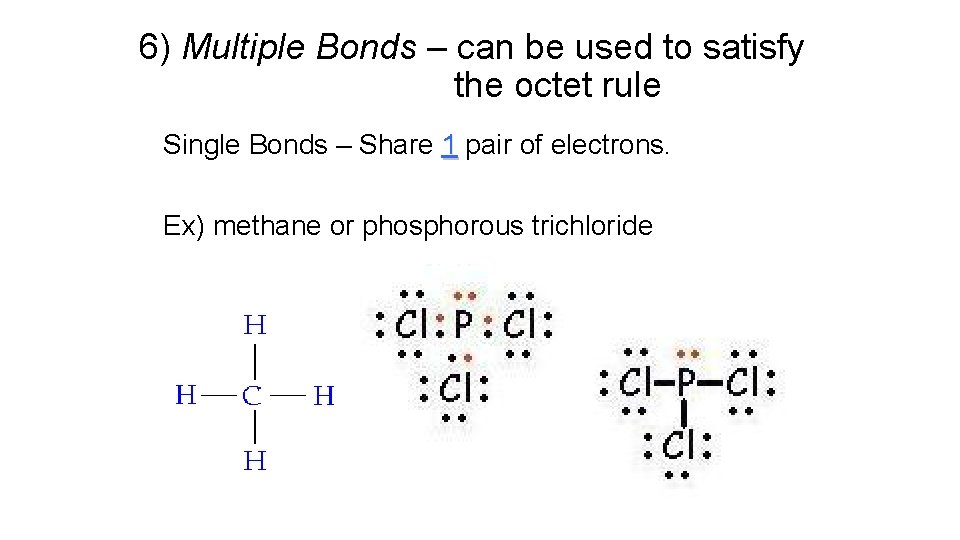
6) Multiple Bonds – can be used to satisfy the octet rule Single Bonds – Share 1 pair of electrons. Ex) methane or phosphorous trichloride
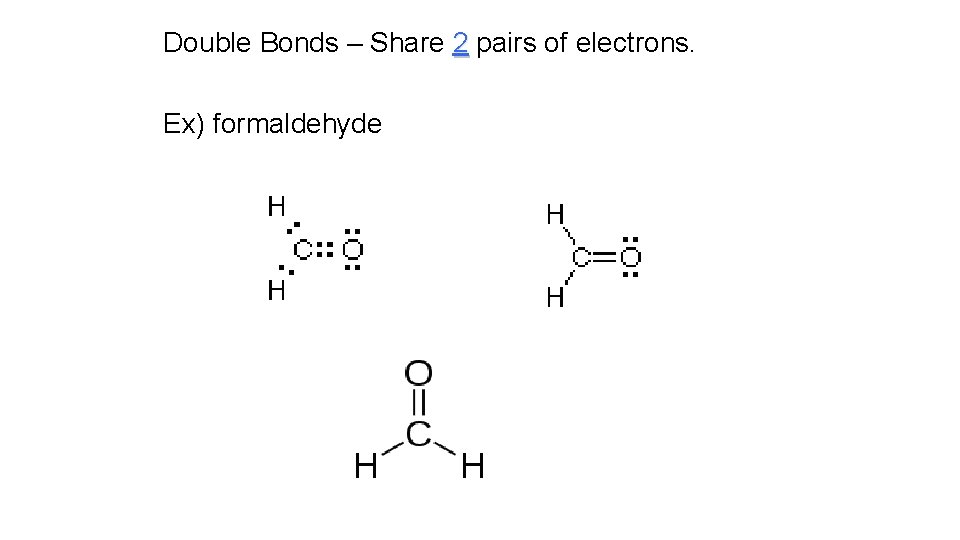
Double Bonds – Share 2 pairs of electrons. Ex) formaldehyde
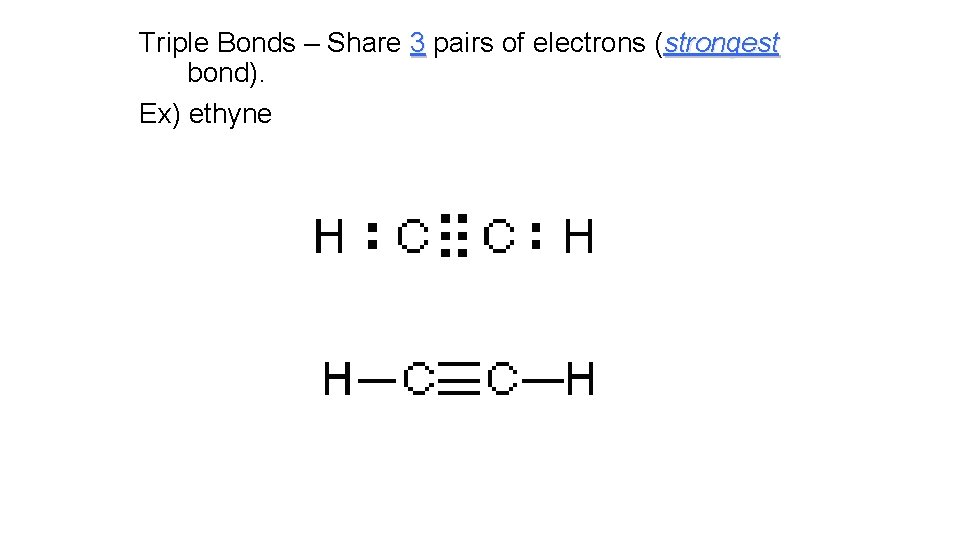
Triple Bonds – Share 3 pairs of electrons (strongest bond). Ex) ethyne
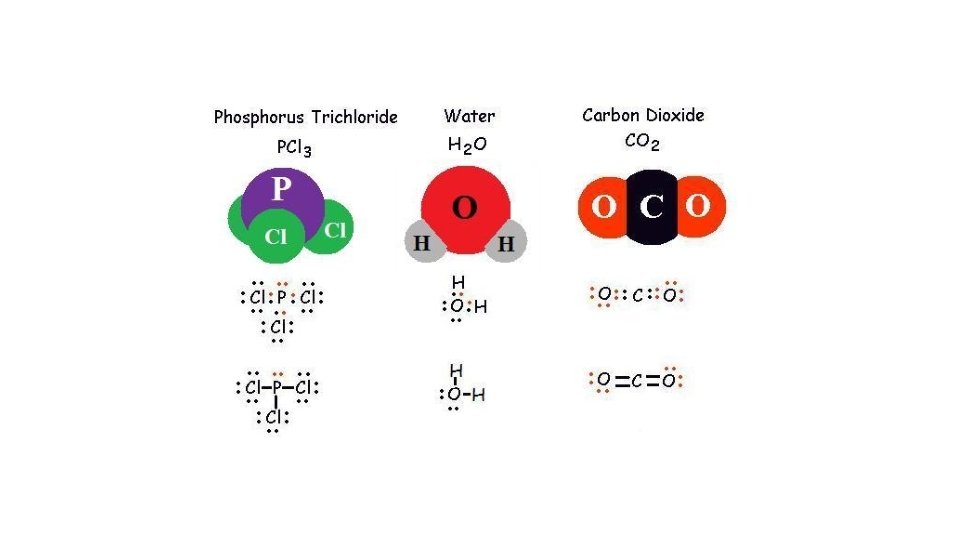
- Slides: 21