Naming Ionic Compounds Binary Compounds Binary Compounds Two

Naming Ionic Compounds

Binary Compounds

Binary Compounds • Two elements are bonded together to form a neutral compound

Notice This works only when the first metal is NOT a transition element Where are the transition metals on the periodic table? Also, compounds including lead and tin cannot be named this way.

Naming Na. Cl 1. Take the name of the 1 st element Sodium 2. Take the prefix of the second element (usually) Sodium chlor 3. Add the ending “ide” Sodium chloride

Examples • KBr • Potassium Bromide • Ca. Cl 2 • Calcium chloride

Naming Ionic Compounds using Polyatomic Ions

Polyatomic Ions Many atoms bonded together as a group with a charge Examples: SO 42 - (Sulfate) OH- (Hydroxide) NH 4+ (Ammonium)

To name Ca(OH)2 1. Take the name of the 1 st element Calcium 2. Find the name of the polyatomic ion and add it to the end Calcium hydroxide
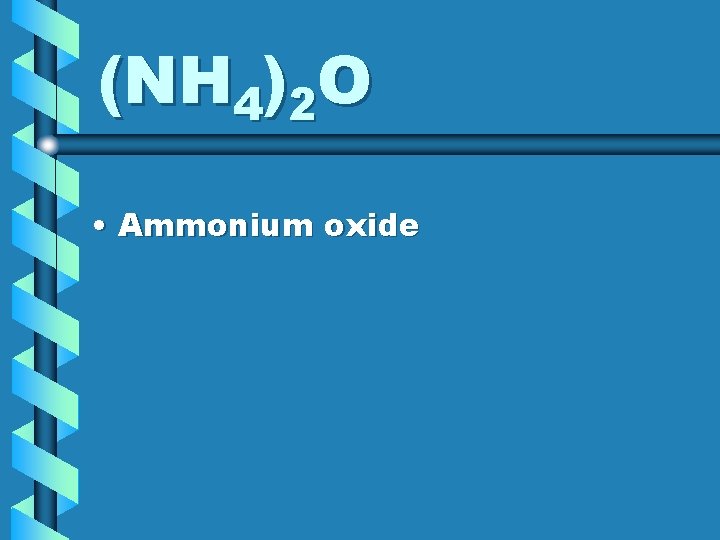
(NH 4)2 O • Ammonium oxide

Practice • Na 2 SO 4 • Sodium sulfate • Ca(NO 3)2 • Calcium nitrate
Naming Ionic Compounds Using Transition Elements

Transition elements (transition metals) often have multiple oxidation numbers • Example Fe 2+ and Fe 3+ To inform others what oxidation state you have, you place the transition element’s oxidation state in parenthesis in Roman Numerals

Roman Numeral Review 1=I 2 = II 3 = III 4 = IV 5=V 6 = VI 7 = VII 8 = VIII

To determine transition oxidation number 1. Put the cation and anion symbols in parenthesis 2. Add the oxidation number of the anion 3. Multiple it by the number of anions you have

To determine transition oxidation number 4. Take that number and divide it by the number of cations you have 5. The result is the oxidation number with a positive charge for the transition element

Fe. O (Fe)(O) • (Fe)2+(O)2 • Iron (II) oxide (Fe)2(O)3 • Fe+3 and O 2 • Iron (III) oxide

Practice Ag. Cl • Silver (I) chloride Hg. Br 2 • Mercury (II) bromide

Transition element and polyatomic ion • Co(NO 3)2 Step 1: (Co)(NO 3)2 Step 2: Co(NO 3)2 -1 Step 3: -1 times 2 is -2 Step 4: Take the 2 and divide it by the subscript 1 on Co and it is 2 Step 5: The oxidation number is 2 for Co • Cobalt (II) nitrate
- Slides: 19