NAMING IONIC COMPOUNDS 3 TYPES OF IONIC COMPOUNDS










- Slides: 19

NAMING IONIC COMPOUNDS

3 TYPES OF IONIC COMPOUNDS TO NAME 1) Metal ion to nonmetal ion • Cation: metal Anion: nonmetal 2) Metal ion to polyatomic ion • Cation: metal Anion: polyatomic 3) Polyatomic ion to polyatomic ion • Cation: polyatomic **some metals are special** Anion: polyatomic

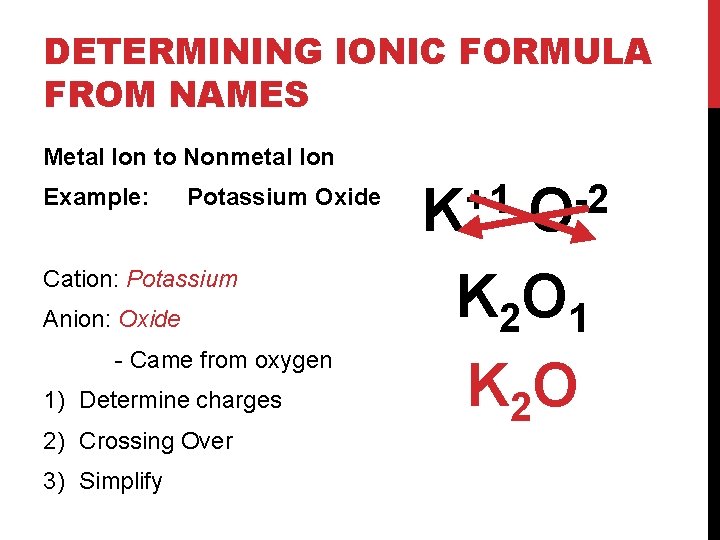
DETERMINING IONIC FORMULA FROM NAMES Metal Ion to Nonmetal Ion Example: Potassium Oxide Cation: Potassium Anion: Oxide - Came from oxygen 1) Determine charges 2) Crossing Over 3) Simplify +1 K -2 O K 2 O 1 K 2 O

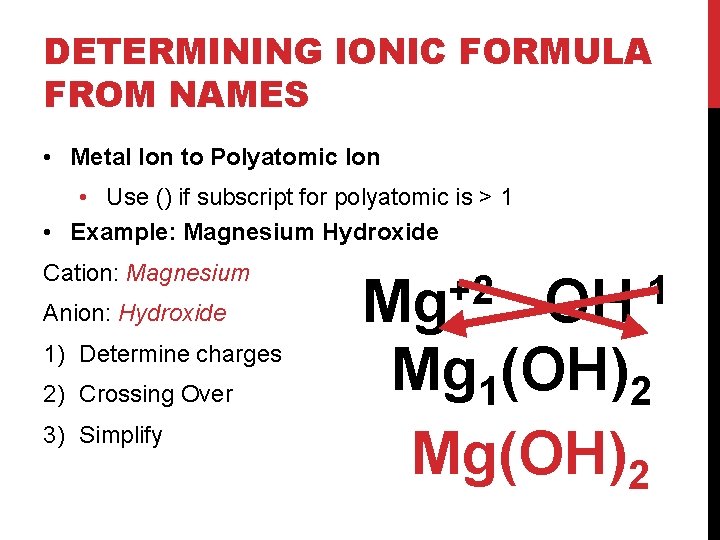
DETERMINING IONIC FORMULA FROM NAMES • Metal Ion to Polyatomic Ion • Use () if subscript for polyatomic is > 1 • Example: Magnesium Hydroxide Cation: Magnesium Anion: Hydroxide 1) Determine charges 2) Crossing Over 3) Simplify +2 Mg -1 OH Mg 1(OH)2 Mg(OH)2

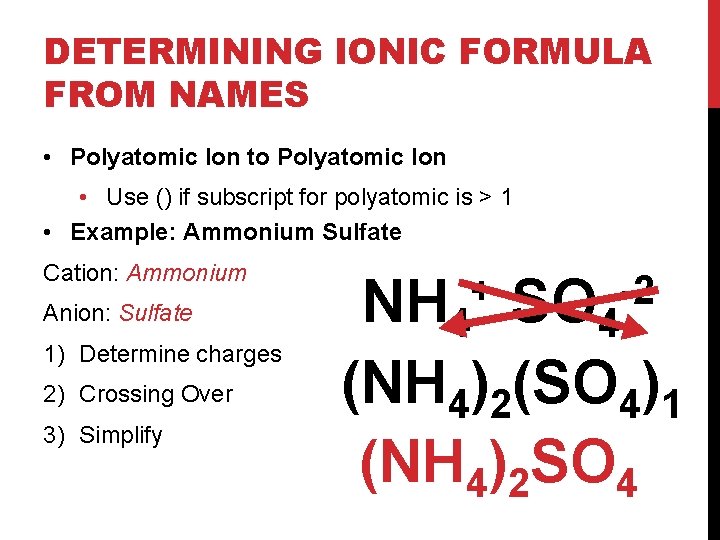
DETERMINING IONIC FORMULA FROM NAMES • Polyatomic Ion to Polyatomic Ion • Use () if subscript for polyatomic is > 1 • Example: Ammonium Sulfate Cation: Ammonium Anion: Sulfate 1) Determine charges 2) Crossing Over 3) Simplify NH 4 SO 4 (NH 4)2(SO 4)1 (NH 4)2 SO 4 + -2

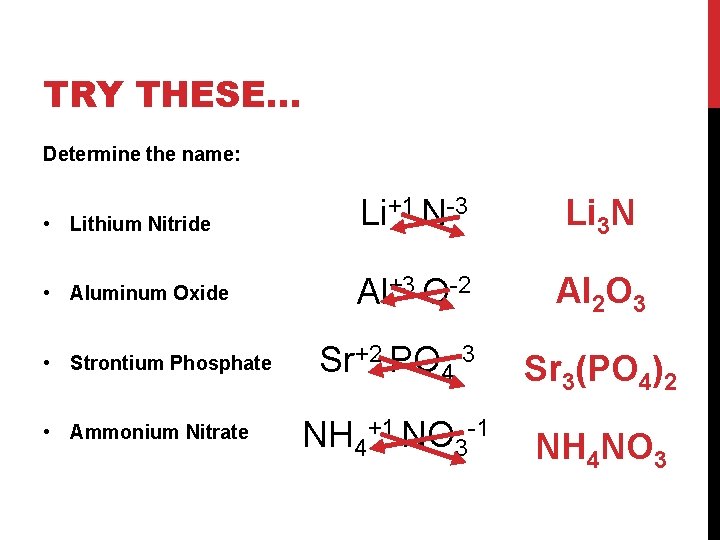
TRY THESE… Determine the name: • Lithium Nitride Li+1 N-3 Li 3 N • Aluminum Oxide Al+3 O-2 Al 2 O 3 • Strontium Phosphate • Ammonium Nitrate Sr+2 PO 4 -3 Sr 3(PO 4)2 NH 4+1 NO 3 -1 NH 4 NO 3

RULES FOR NAMING IONIC COMPOUNDS • Write name of metal (no change to name) • Never change name of polyatomic • Nonmetal endings change to –ide • Sulfur (S) becomes sulfide (S-2) **some metals are special**

NAMING IONIC COMPOUNDS Metal ion to nonmetal ion Example: Mg. S Cation (metal): Magnesium Anion (nonmetal): Sulfur - Turns into Sulfide Magnesium Sulfide

NAMING IONIC COMPOUNDS Metal ion to polyatomic ion Example: Ca. CO 3 Cation (metal): Calcium Anion (polyatomic): Carbonate Calcium Carbonate

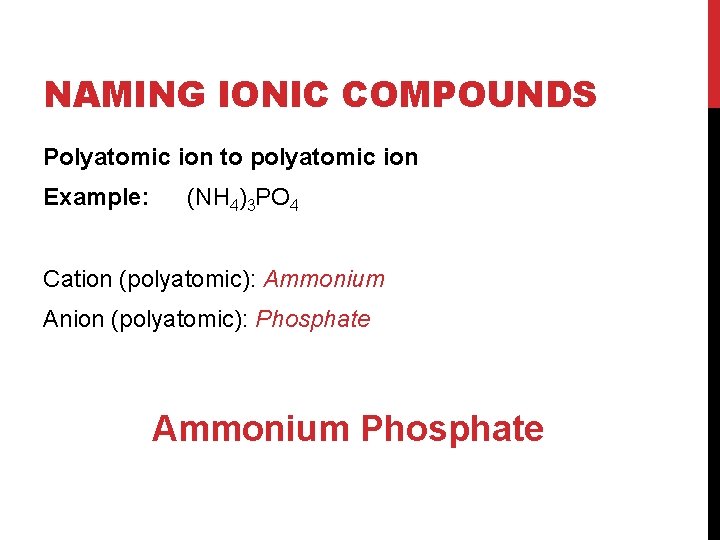
NAMING IONIC COMPOUNDS Polyatomic ion to polyatomic ion Example: (NH 4)3 PO 4 Cation (polyatomic): Ammonium Anion (polyatomic): Phosphate Ammonium Phosphate

TRY THESE… Determine the name: • Chlorine & Lithium Chloride • Potassium & Oxygen Potassium Oxide • Calcium & Carbonate Calcium Carbonate • Sulfate & Ammonium Sulfate

NAMING SPECIAL METAL IONIC COMPOUNDS

SPECIAL METALS • Transition Metals, Tin (Sn) & Lead (Pb) are ‘special’ metals • These metals can create more than one cation • The cation is distinguished by a roman numeral (I, III, or IV) • Ex: Cu 3 N 2 Copper (II) Nitride

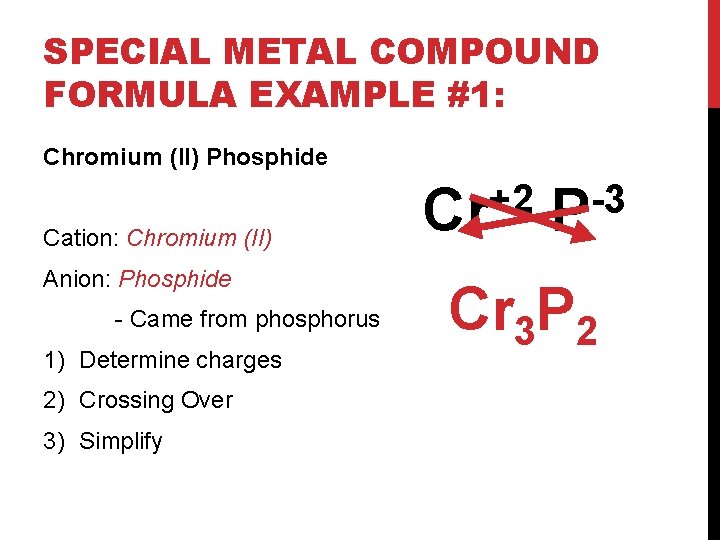
SPECIAL METAL COMPOUND FORMULA EXAMPLE #1: Chromium (II) Phosphide Cation: Chromium (II) Anion: Phosphide - Came from phosphorus 1) Determine charges 2) Crossing Over 3) Simplify +2 Cr -3 P Cr 3 P 2

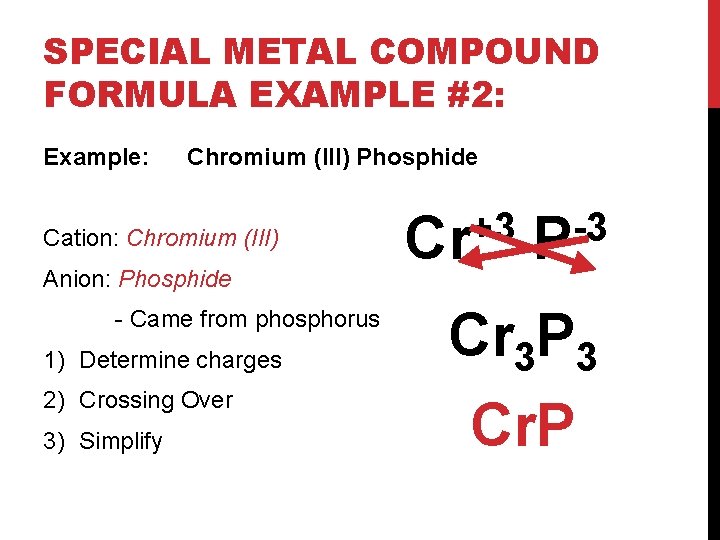
SPECIAL METAL COMPOUND FORMULA EXAMPLE #2: Example: Chromium (III) Phosphide Cation: Chromium (III) Anion: Phosphide - Came from phosphorus 1) Determine charges 2) Crossing Over 3) Simplify +3 Cr -3 P Cr 3 P 3 Cr. P

TRY THESE… Determine the name: • Copper (II) chloride Cu. Cl 2 • Manganese (III) sulfide Mn 2 S 3 • Tin (IV) Phosphate Sn 3(PO 4)4 • Lead (II) Nitrate Pb(NO 3)2

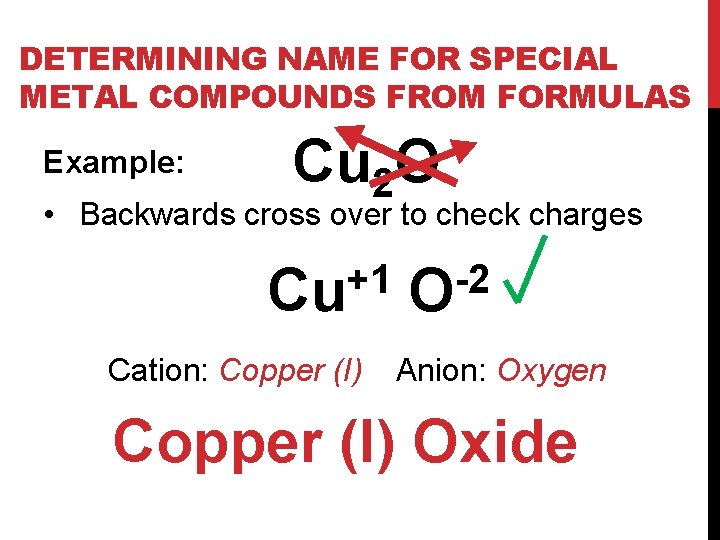
DETERMINING NAME FOR SPECIAL METAL COMPOUNDS FROM FORMULAS Example: Cu 2 O • Backwards cross over to check charges +1 Cu Cation: Copper (I) -2 O Anion: Oxygen Copper (I) Oxide

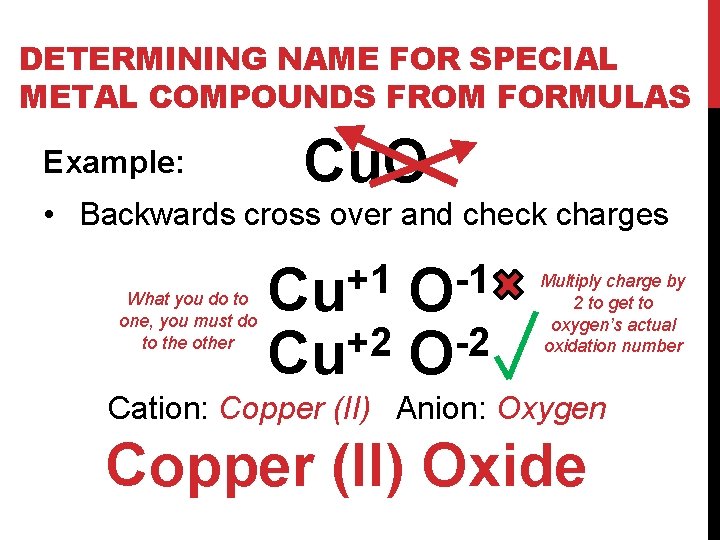
DETERMINING NAME FOR SPECIAL METAL COMPOUNDS FROM FORMULAS Example: Cu. O • Backwards cross over and check charges What you do to one, you must do to the other +1 Cu +2 Cu -1 O -2 O Multiply charge by 2 to get to oxygen’s actual oxidation number Cation: Copper (II) Anion: Oxygen Copper (II) Oxide

TRY THESE… Determine the name: • Cr 2 O 3 Chromium (III) Oxide • Co. F 2 Cobalt (II) Fluoride • Fe(NO 3)3 • Sn. SO 4 Iron (III) Nitrate Tin (II) Sulfate