Naming Ionic and Covalent Binary Compounds Cs 2

Naming Ionic and Covalent Binary Compounds!!

Cs 2 S 1. Name it 2. Ionic or covalent?

Cesium Sulfide Ionic

CO 1. Name it 2. Ionic or covalent?

Carbon monoxide Covalent

Ca(NO 3)2 1. Name it 2. Ionic or covalent?

Calcium Nitrate Ionic

Lithium nitrate Write the formula

Li. NO 3

Strontium Carbonate Write the formula

Sr(CO 3)

Aluminum sulfate Write the formula

Al 2(SO 4)3

Fe. S Name it

Iron (II) sulfide

Cu. Br Name it

Copper (I) bromide
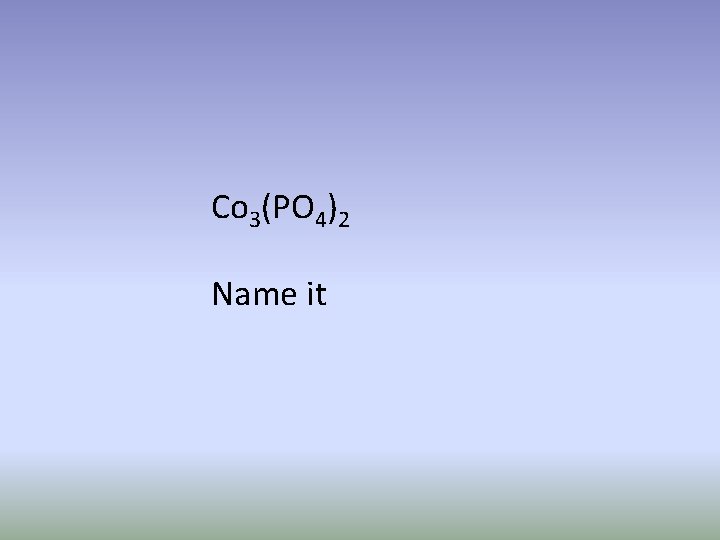
Co 3(PO 4)2 Name it

Cobalt (II) phosphate

Manganese (IV) phosphate Write the formula
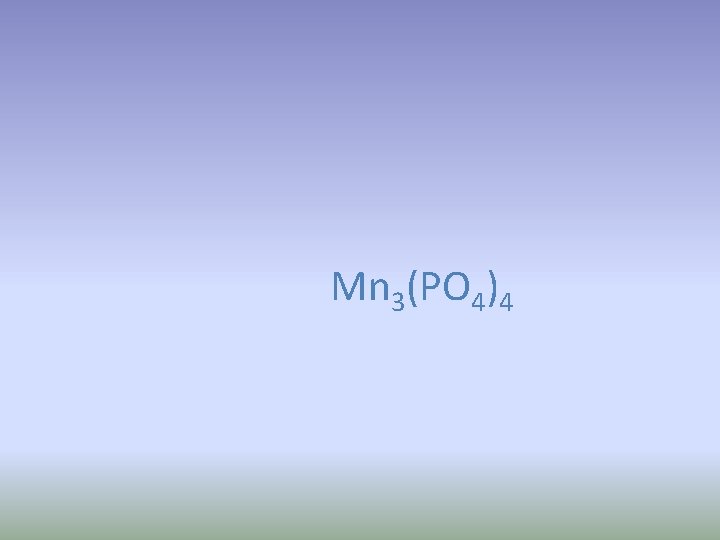
Mn 3(PO 4)4

B 2 O 3 1. Name it 2. Ionic or covalent?

Diboron trioxide Covalent

Ti. Cl 4 1. Name it 2. Ionic or covalent?

Titanium (IV) chloride Ionic

K 3 P 1. Name it 2. Ionic or covalent?

Potassium phosphide Ionic

OF 2 1. Name it 2. Ionic or covalent?

Oxygen difluoride Covalent

NF 3 1. Name it 2. Ionic or covalent?

Nitrogen trifluoride Covalent

Cu. Cl 1. Name it 2. Ionic or covalent?

Copper (I) chloride Ionic
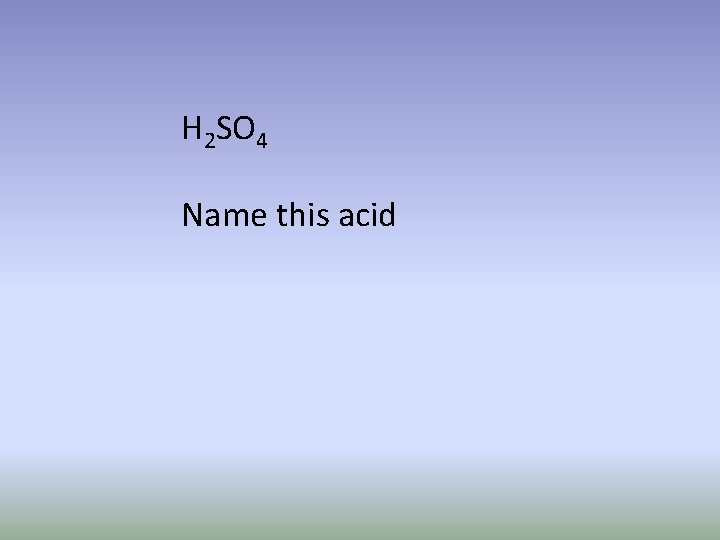
H 2 SO 4 Name this acid

Sulfuric acid

B 2 H 6 1. Name it 2. Ionic or covalent?

Diboron hexahydride Covalent

Phosphorous trichloride 1. Write the formula 2. Ionic or covalent?

PCl 3 Covalent

Carbon monofluoride 1. Write the formula 2. Ionic or covalent?

CF Covalent

Hydrofluoric acid Write the formula for this acid

HF

KOH Name this base

Potassium hydroxide

Ammonium sulfite 1. Write the formula 2. Ionic or covalent?
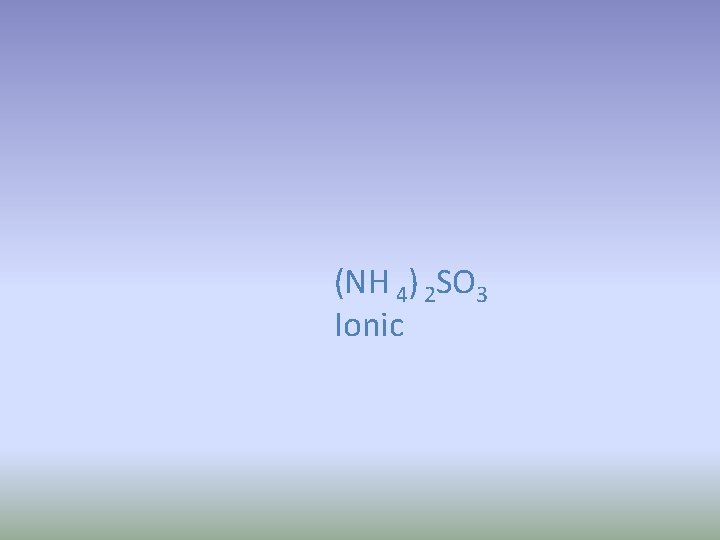
(NH 4) 2 SO 3 Ionic

Carbon tetrabromide 1. Write the formula 2. Ionic or covalent?

CBr 4 Covalent

Magnesium hydroxide 1. Write the formula 2. Classify (ionic, covalent, acid, base)
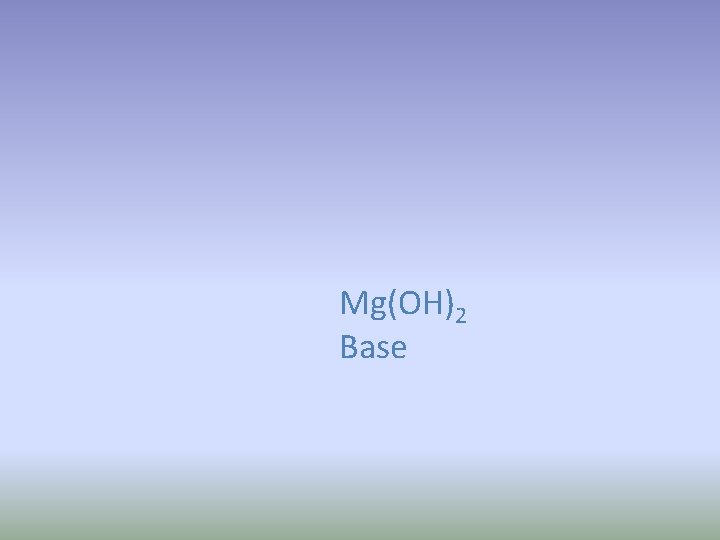
Mg(OH)2 Base

Titanium (IV) bromide 1. Write the formula 2. Ionic or covalent?

Ti. Br 4 Ionic

Nitrous acid Write the formula for this acid

HNO 2

Dinitrogen pentoxide 1. Write the formula 2. Ionic or covalent?

N 2 O 5 Covalent

Phosphoric acid Write the formula
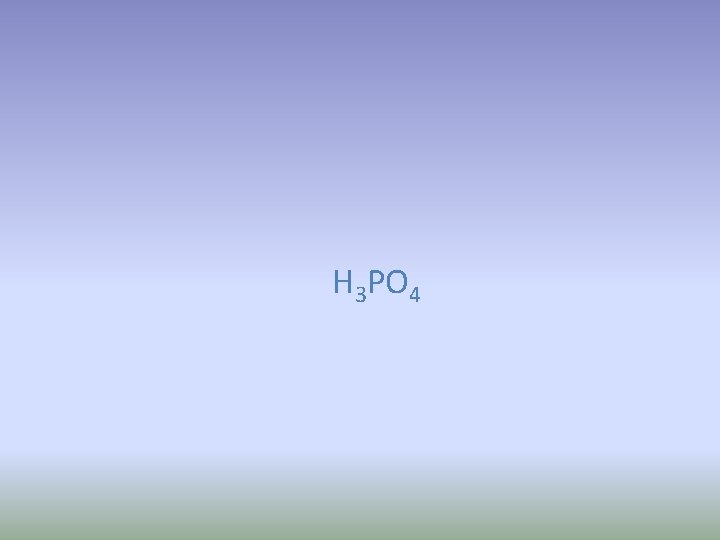
H 3 PO 4

Congratulations!! You may now consider yourself a binary/tertiary compound naming Master!!!!
- Slides: 60