Naming Covalent Molecules n n Binary molecular compounds
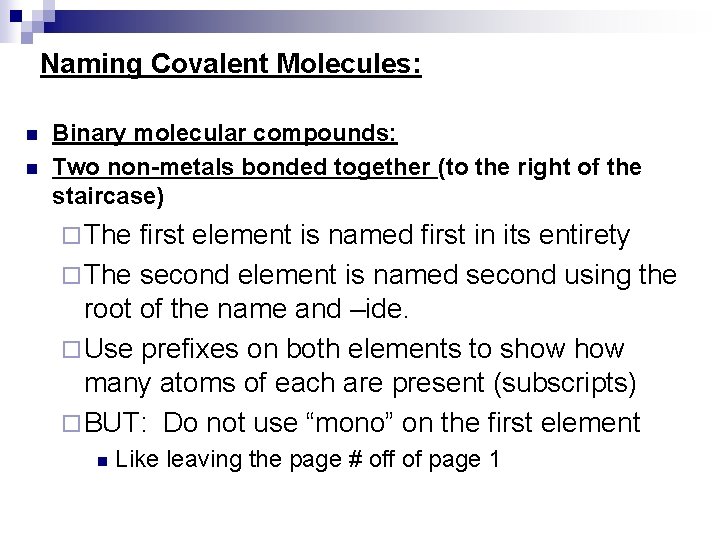
Naming Covalent Molecules: n n Binary molecular compounds: Two non-metals bonded together (to the right of the staircase) ¨ The first element is named first in its entirety ¨ The second element is named second using the root of the name and –ide. ¨ Use prefixes on both elements to show many atoms of each are present (subscripts) ¨ BUT: Do not use “mono” on the first element n Like leaving the page # off of page 1

Prefixes # Atoms Prefix 1 Mono 6 Hexa 2 Di 7 Hepta 3 Tri 8 Octa 4 Tetra 9 Nona 5 Penta 10 Deca

Examples Formula Common Name Molecular Compound Name H 2 O Water Dihydrogen monoxide NH 3 Ammonia Nitrogen trioxide N 2 H 4 Hydrazine Dinitrogen tetrahydride N 2 O Nitrous oxide Dinitrogen monoxide NO Nitric oxide Nitrogen monoxide

Naming Formulas 1. 2. 3. 4. Name the first element with prefix (if more than 1 is present) Get the root name of the second element Put a prefix (if more than 1 is present) on the root Add “ide” to the end of the second element.

Writing Formulas for Covalent Compounds 1. 2. 3. 4. 5. Get first name from symbol in periodic table. For second element, remove “ide” and find symbol in periodic table using root of the name. If there are prefixes, use them as subscripts (eg: “di” would be “ 2”) Example: Carbon Dioxide CO 2
- Slides: 5