Naming Covalent Compounds Covalent Bonds Sharing of electrons

Naming Covalent Compounds
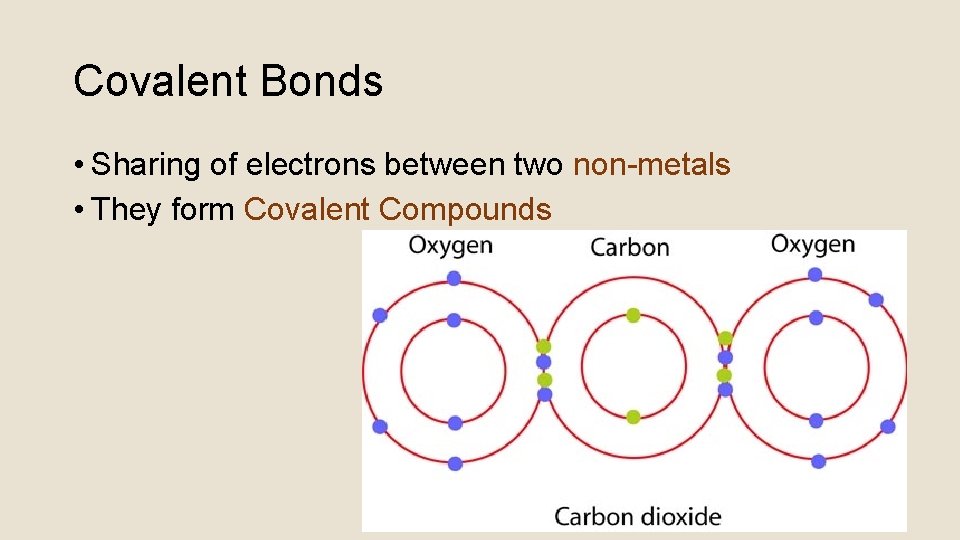
Covalent Bonds • Sharing of electrons between two non-metals • They form Covalent Compounds
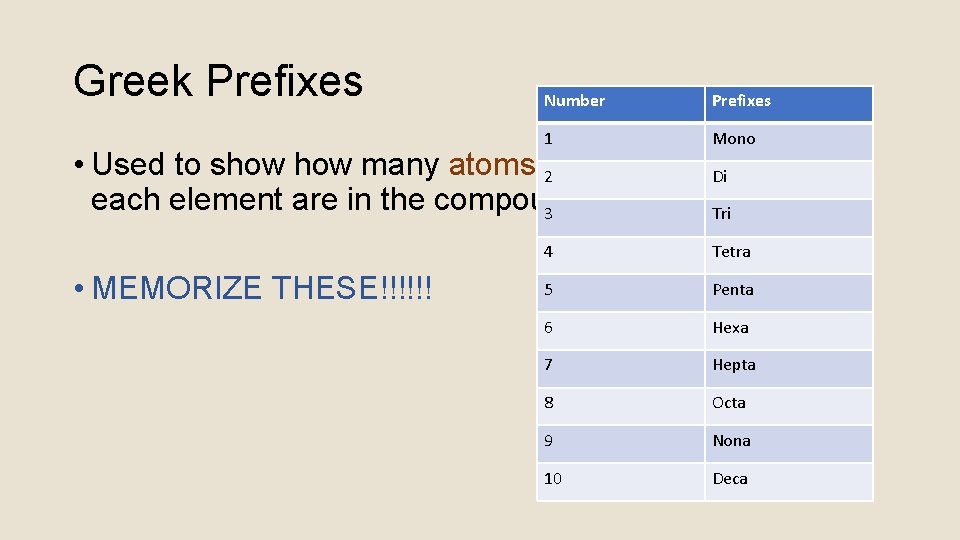
Greek Prefixes Number Prefixes 1 Mono • Used to show many atoms 2 of each element are in the compound 3 • MEMORIZE THESE!!!!!! Di Tri 4 Tetra 5 Penta 6 Hexa 7 Hepta 8 Octa 9 Nona 10 Deca

Writing Formulas for Covalent Compounds • Write the symbol of the first element, followed by the subscript corresponding to its prefix • Repeat for the second element • Example: diphosporous trioxide • 2 phosphorous, 3 oxygen = P 2 O 3

Writing Names for Covalent Compounds • Write the name of the first element with the correct prefix before it • If the subscript is “ 1”, no prefix is needed • Write the name of the second element with the correct prefix before it, and change the ending to “ide” • Here the mono prefix is needed!

Writing Names for Covalent Compounds • Example: • ICl • I = iodine (don’t put the “mono”) • Cl = monochloride • iodine monochloride

Try These: • S 4 N 2 • Br. F • P 2 O 6 • tetraphosphorus trisulphide • trisilicon tetranitride • oxygen difluoride
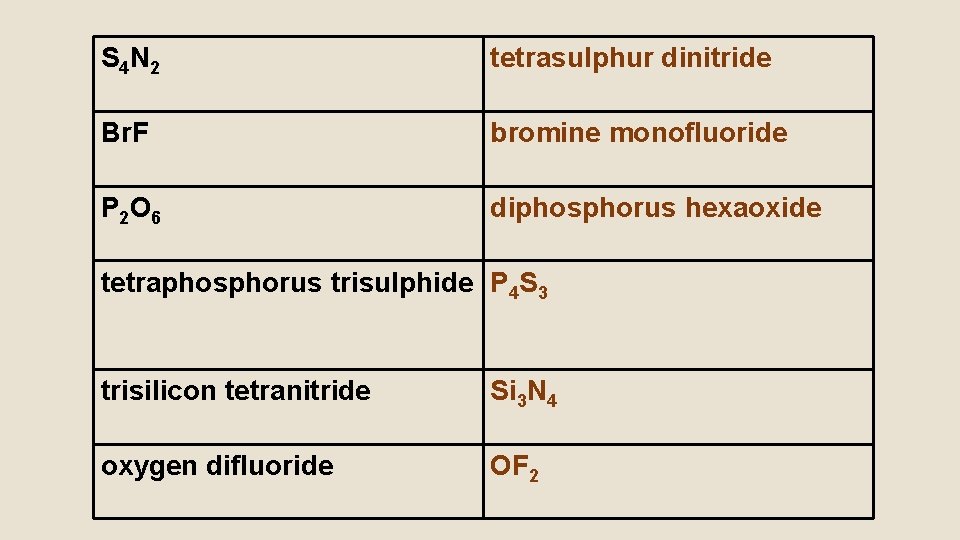
S 4 N 2 tetrasulphur dinitride Br. F bromine monofluoride P 2 O 6 diphosphorus hexaoxide tetraphosphorus trisulphide P 4 S 3 trisilicon tetranitride Si 3 N 4 oxygen difluoride OF 2

Naming Hydrates: • Hydrate: an ionic salt that has water associated with it (incorporated into the crystal lattice structure) • Named just like ionic compounds, except… 1. the Greek prefixes are added at the end with the word “hydrate” to show many water molecules are present 2. A dot is added between the formula of the salt and the formula of the water
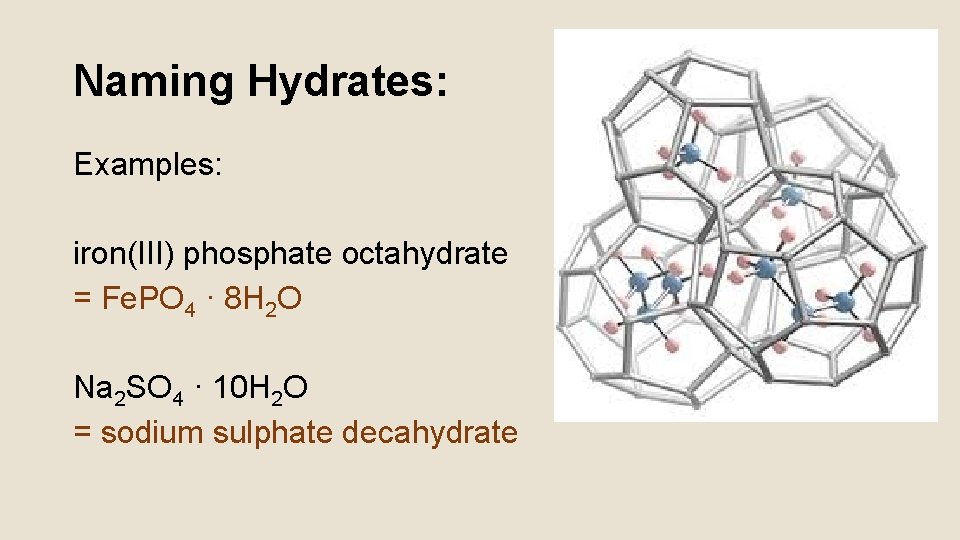
Naming Hydrates: Examples: iron(III) phosphate octahydrate = Fe. PO 4 · 8 H 2 O Na 2 SO 4 · 10 H 2 O = sodium sulphate decahydrate
- Slides: 10