NAMING COVALENT COMPOUNDS BINARY MOLECULAR COMPOUNDS Binary molecular

NAMING COVALENT COMPOUNDS

BINARY MOLECULAR COMPOUNDS Binary molecular compounds are formed from two nonmetals held together by covalent bonds Elements in binary molecular compounds can combine in different ways. Carbon and Oxygen can combine as CO and CO 2
NAMING MOLECULAR COMPOUNDS Covalent compounds are named differently than ionic compounds Since covalent compounds can form multiple compounds with each other they can not be named the same as ionic compounds Nitrogen and oxygen can form N 2 O, No 2, and N 2 O 5. If they were named like ionic compounds they would all be called nitrogen oxide.

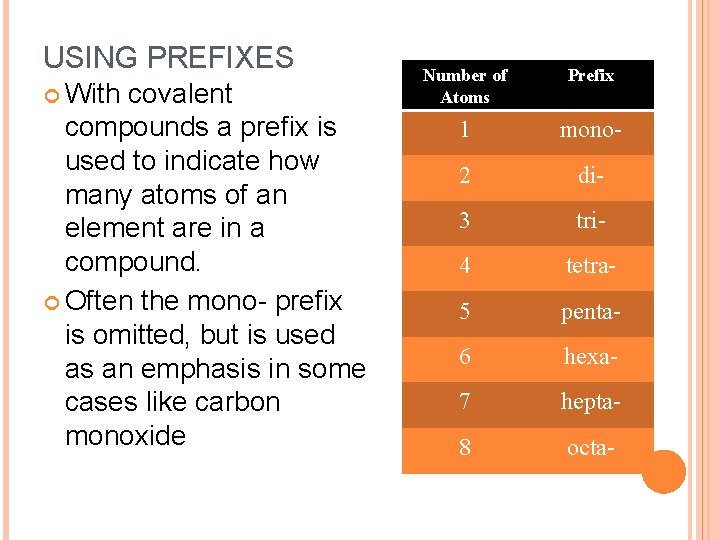
USING PREFIXES With covalent compounds a prefix is used to indicate how many atoms of an element are in a compound. Often the mono- prefix is omitted, but is used as an emphasis in some cases like carbon monoxide Number of Atoms Prefix 1 mono- 2 di- 3 tri- 4 tetra- 5 penta- 6 hexa- 7 hepta- 8 octa-
NAMING MOLECULAR COMPOUNDS First confirm that both elements are nonmetals Name the elements in the order listed in the formula Use prefixes to indicate the number and kind of atoms The suffix of the second element is –ide
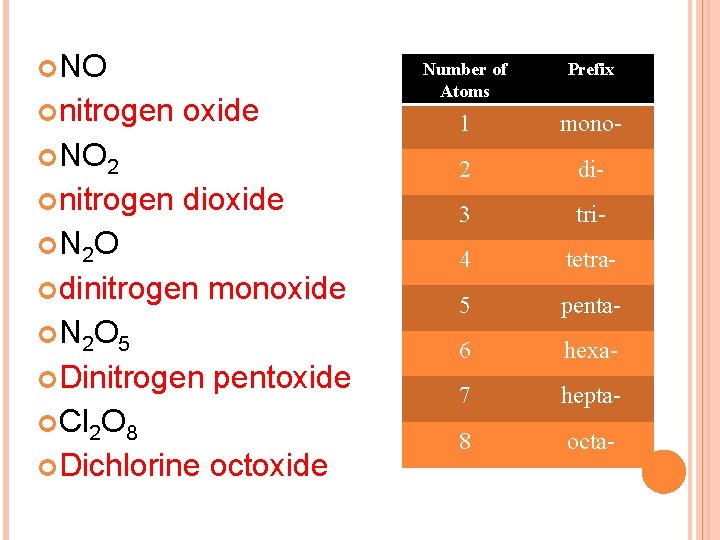
NO nitrogen oxide NO 2 nitrogen dioxide N 2 O dinitrogen monoxide N 2 O 5 Dinitrogen pentoxide Cl 2 O 8 Dichlorine octoxide Number of Atoms Prefix 1 mono- 2 di- 3 tri- 4 tetra- 5 penta- 6 hexa- 7 hepta- 8 octa-
WRITING BINARY MOLECULAR COMPOUNDS When writing the formula for a binary molecular compound, use the prefixes in the name to give the numbers of each element in the compound First write the symbols of the elements involved in the compound Next add subscripts based on the prefixes

EXAMPLE Write the formula for silicon carbide The symbol for silicon is Si and the symbol for carbon is C There are no prefixes, so the formula is Si. C

EXAMPLE Write the formula for carbon dioxide The symbol for carbon is C and the symbol for oxygen is O The prefix on oxygen is di- which means 2, so the formula is CO 2
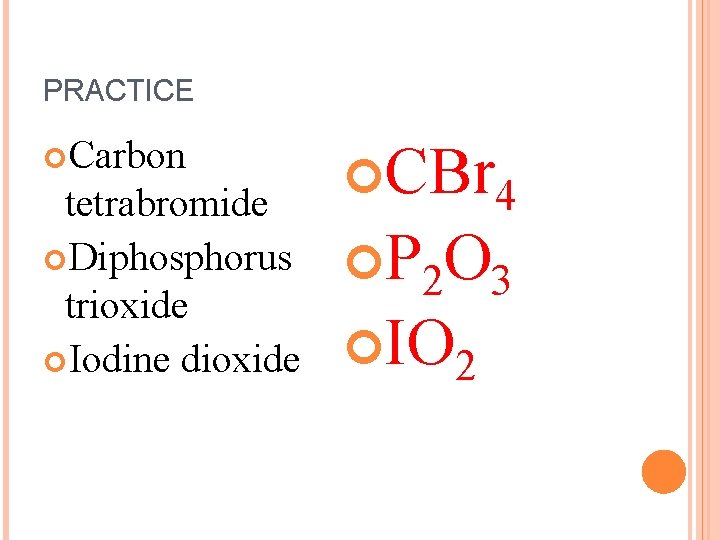
PRACTICE Carbon tetrabromide Diphosphorus trioxide Iodine dioxide CBr 4 P 2 O 3 IO 2
LAWS OF DEFINATE PROPORTION The law of definite proportions state that in the sample of any compound, the masses of the elements are always in the same proportions No matter the size of the sample, the ratio of mass of one compound to another will always be the same
The law of multiple proportions states that whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers. When comparing to compounds with the same elements, the ratio of masses will be of the elements will be in a whole number ratio. H 20 and H 202, the ratio of oxygen is 2: 1 for the same mass of hydrogen
- Slides: 13