NAMING COMPOUNDS The chemical formula represents the composition














- Slides: 22

NAMING COMPOUNDS The chemical formula represents the composition of each molecule. In writing the chemical formula, in almost all cases the element farthest to the left of the periodic table is written first because it is the least electronegative.

NAMING COMPOUNDS So for example the chemical formula of a compound that contains one sulfur atom and six fluorine atoms is SF 6. If the two elements are in the same Group or Family, the symbol of the element of that is lower in the group (i. e. heavier & lower electronegativity) is written first e. g. IF 3.

Naming Covalent Compounds In naming covalent compounds, the name of the first element in the formula is unchanged. The suffix “-ide” is added to the second element. Often a prefix on the name of the second element indicates the number of the element in the compound SF 6 – sulfur hexafluoride P 4 O 10 – tetraphosphorous decoxide CO – carbon monoxide CO 2 – carbon dioxide

Naming Covalent Compounds Cont. ØSF 6 – sulfur hexafluoride ØP 4 O 10 – tetraphosphorous decoxide ØCO – carbon monoxide ØCO 2 – carbon dioxide ØNotice that the first element does NOT get a mono- prefix!

Hydrogen Cmpds The binary compounds of hydrogen are special cases. They Example were discovered before a HF - hydrogen convention was adopted and fluoride hence their original names have HCl - hydrogen stayed Water H 2 O is not called dihydrogen monoxide Hydrogen forms binary compounds with almost all nonmetals except the noble gases. chloride H 2 S - hydrogen sulfide

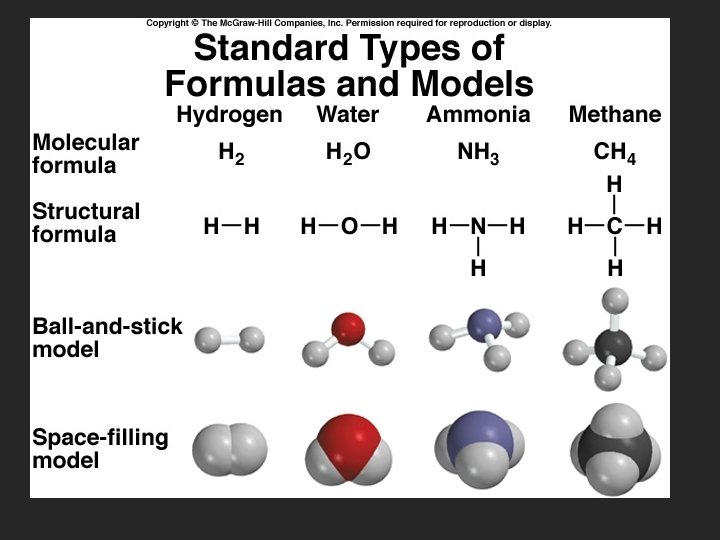
Organic Nomenclature Organic The molecular formulas for molecules compounds (containing C) containing C and H (called have a hydrocarbons) separate are written with C nomenclature first. Example, CH 4, C 2 H 6, etc.

BINARY IONIC COMPOUNDS Compounds formed by elements on opposite sides of the periodic table which either give up (left side = metals) or take up electrons (right side = nonmetals). Depending on the atom, there can be an exchange of more than one electron resulting in charges greater than ± 1.

Group (Ca +2 ) Group Group IA – alkali metals – loose 1 e - to form +1 (Na + ) II A– alkaline earth metals –loose 2 e - to form +2 III A– loose three e - to form +3 (Al +3 ) IV A– loose four e - to form +4 (Sn +4 ) V A– accept three e - to form – 3 (N -3 ) VI A– accept two e - to form – 2 (O -2 ) VIIA – accept one e - to form – 1 (Cl -1 )

Naming BINARY IONIC compounds Cations For Na+, Ca 2+, the name of the ion is the same except refer to the ion. So SODIUM ION or SODIUM CATION Anions – suffix – “ide” So Cl- is chloride, Oxygen O 2 - is OXIDE & S 2 - is SULFIDE Na. Cl - sodium chloride Ca. Cl 2 - calcium chloride

Naming BINARY IONIC compounds EXAMPLES CONT. Ba. Cl 2 barium chloride K 2 O Mg(OH)2 potassium oxide magnesium hydroxide KNO 3 potassium nitrate

Covalent, charged compounds MOLECULAR IONS Negative Molecular Ions Positive Molecular Ions End the name with “ium” or “onium” NH 4+ is ammonium, H 3 O + is hydronium - NO 3 - NITRATE 2 - SO 4 - SULFATE - NO 2 - NITRITE 3 - PO 4 - PHOSPHATE

Transition Elements The transition elements are chemically quite different from the metals in the “A” block, due to differences in electronic configuration For example, Fe can loose two or three electrons to become Fe 2+ and Fe 3+ , respectively.

To identify the charge of Fe in a compound the following nomenclature is used. Fe 2+ is iron(II) Fe 3+ is iron (III) So iron(III) chloride is Fe. Cl 3 An older scheme differentiated between the lower and higher charge by ending the name of the element with “ous” to indicate the lower charge and “ic” for the higher. ferrous chloride => Fe. Cl 2 ferric chloride => Fe. Cl 3 However, this convention does not indicate the numerical value of the charge.

TERTIARY IONIC COMPOUNDS NAME THE METAL NORMALLY, WITH A ROMAN NUMERAL IF NEEDED FOR A TRANSITION METAL NAME THE APROPRIATE POLYATOMIC ION Au 2(Cr 2 O 7)3 Mg (SCN)2 Gold (III) Dichromate Magnesium Thiocyanate Sc 4(P 2 O 7)3 V 3(BO 3)5 Scandium Pyrophosphate Vanadium (V) Borate

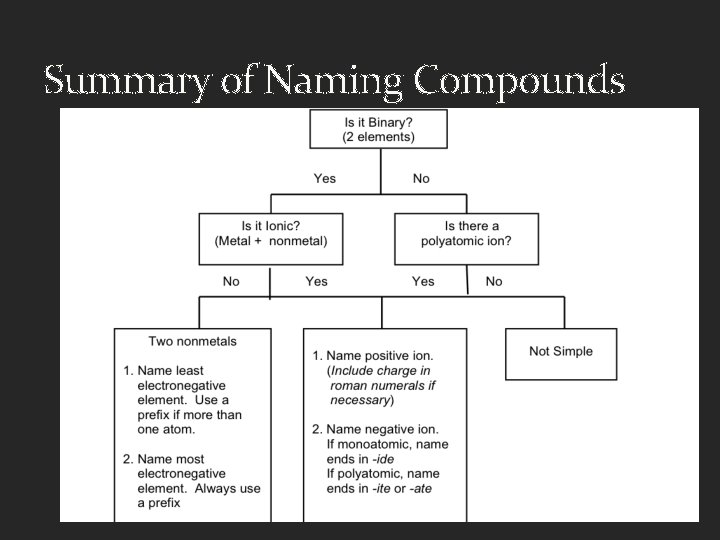
Summary of Naming Compounds

NAMING ACIDS An acid can be defined as a substance that yields hydrogen ions (H+) when dissolved in water. EXAMPLE: HCl • Pure substance, hydrogen chloride • Dissolved in water (H+ Cl-), hydrochloric acid • An oxoacid is an acid that contains hydrogen, oxygen, and another element. HNO 3 nitric acid H 2 CO 3 carbonic acid H 2 SO 4 sulfuric acid


Naming Acids Cont. Name of ion Name of Acid Sulfate = (SO 4)-2 Sulfuric Acid = H 2 SO 4 Sulfite = (SO 3)-2 Sulfurous Acid = H 2 SO 3 Hyposulfite = Doesn’t exist! (SO 2)-2

Representing Formulas of Covalent & Ionic Bonds


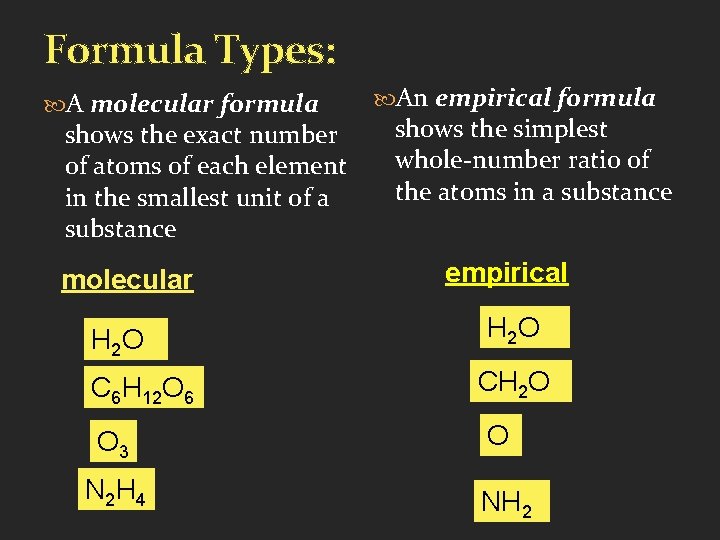
Formula Types: A molecular formula shows the exact number of atoms of each element in the smallest unit of a substance molecular H 2 O C 6 H 12 O 6 O 3 N 2 H 4 An empirical formula shows the simplest whole-number ratio of the atoms in a substance empirical H 2 O CH 2 O O NH 2

This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Revisions and additions by A. Vasto