Naming Compounds cations and anions Elements and symbols

Naming Compounds, cations and anions

Elements and symbols that you should know: Part 1 – The obvious ones: 1) Hydrogen H 8) Oxygen O 2) Helium He 9) Fluorine F 3) Lithium Li 10) Neon Ne 4) Beryllium Be 11) Magnesium Mg 5) Boron B 12) Aluminium Al 6) Carbon C 13) Silicon Si 7) Nitrogen N 14) Phosphorus P

Some more obvious ones: 15) Sulphur S 18) Calcium Ca 16) Chlorine Cl 19) Zinc Zn 17) Argon Ar The less obvious ones: 1) Sodium Na 6) Tin Sn 2) Potassium K 7) Gold Au 3) Iron Fe 8) Mercury Hg 4) Copper Cu 9) Lead Pb 5) Silver Ag

Polyatomic ions (-1 charge) • • • • H 2 PO 4 C 2 H 3 O 2 HSO 3 HCO 3 NO 2 NO 3 CN OH Mn. O 4 Cl. O 2 Cl. O 3 Cl. O 4 Di. Hydrogen phosphate Acetate Hydrogen Sulfite Hydrogen Carbonate Nitrite Nitrate Cyanide Hydroxide Permanganate Hypochlorite Chlorate Perchlorate

Polyatomic ions (-2 charge) • • HPO 4 C 2 O 4 SO 3 SO 4 CO 3 Cr. O 4 Cr 2 O 7 Si. O 3 Hydrogen Phosphate Oxalate Sulfite Sulfate Carbonate Chromate Dichromate Silicate

Polyatomic ions (-3 charge) • PO 3 • PO 4 Phosphite Phosphate
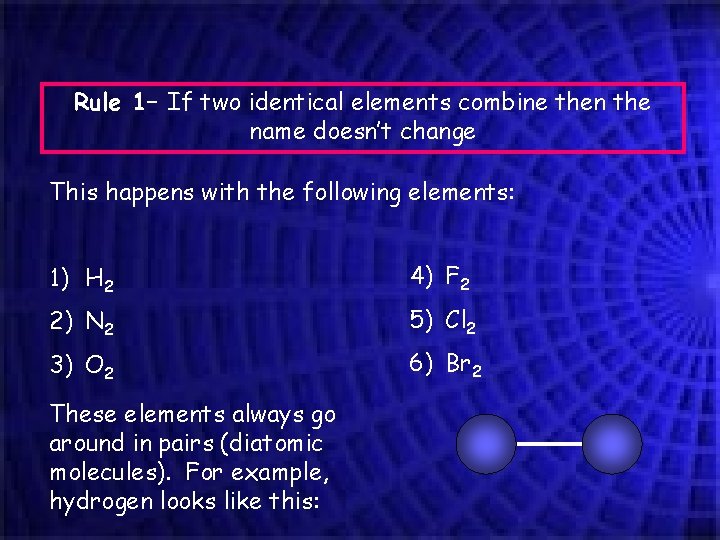
Rule 1– If two identical elements combine then the name doesn’t change This happens with the following elements: 1) H 2 4) F 2 2) N 2 5) Cl 2 3) O 2 6) Br 2 These elements always go around in pairs (diatomic molecules). For example, hydrogen looks like this:

Rule 2 – When two elements join and one is a halogen, oxygen or sulphur the name ends with ____ide e. g. Magnesium + oxygen magnesium oxide 1) Sodium + chlorine 6) KBr 2) Magnesium + fluorine 7) Li. Cl 3) Lithium + iodine 8) Ca. O 4) Chlorine + copper 9) Mg. S 5) Oxygen + iron 10) KF

Rule 3 – When three or more elements combine and two of them are hydrogen and oxygen the name ends with hydroxide e. g. Sodium + hydrogen + oxygen Sodium hydroxide 1) Potassium + hydrogen + oxygen 2) Lithium + hydrogen + oxygen 3) Calcium + hydrogen + oxygen 4) Mg(OH)2

Rule 4 – When three or more elements combine and one of them is oxygen the ending is _____ate e. g. Copper + sulphur + oxygen Copper sulphate 1) Calcium + carbon + oxygen 6) Ag. NO 3 2) Potassium + carbon + oxygen 7) H 2 SO 4 3) Calcium + sulphur + oxygen 8) K 2 CO 3 4) Magnesium + chlorine + oxygen 5) Calcium + oxygen + nitrogen
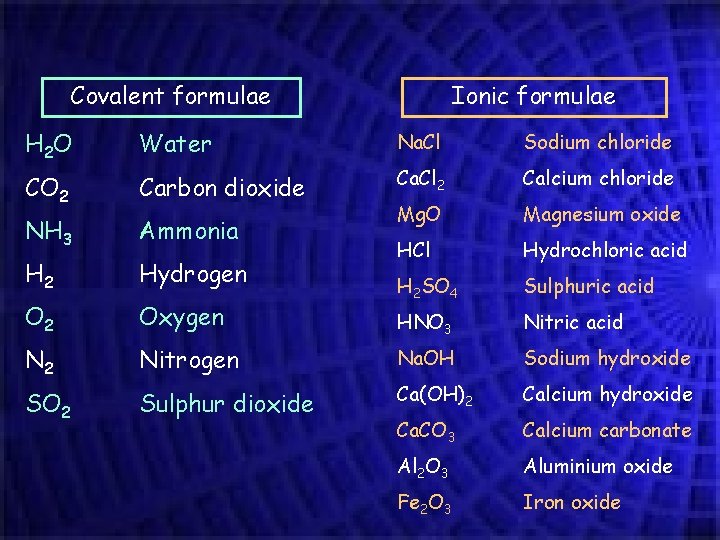
Covalent formulae Ionic formulae H 2 O Water Na. Cl Sodium chloride CO 2 Carbon dioxide Ca. Cl 2 Calcium chloride NH 3 Ammonia Mg. O Magnesium oxide H 2 Hydrogen HCl Hydrochloric acid Sulphuric acid O 2 Oxygen H 2 SO 4 HNO 3 Nitric acid N 2 Nitrogen Na. OH Sodium hydroxide SO 2 Sulphur dioxide Ca(OH)2 Calcium hydroxide Ca. CO 3 Calcium carbonate Al 2 O 3 Aluminium oxide Fe 2 O 3 Iron oxide

This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching.
- Slides: 12