Naming Compounds Binary Ionic Compounds Cation charge is
Naming Compounds Binary Ionic Compounds • Cation (+ charge ) is listed first. • Name of cation is the chemical name. – For many transition metals, the ion is distinguished by the addition of a roman numeral after the chemical name. – Find charge of the anion and choose appropriate roman numeral to balance the charge. • Name of the anion ends in –ide. Examples: • Al 2 O 3 aluminum oxide • Cu. Br 2 copper(II) bromide Mullis 1
Naming Molecular Compounds Binary Molecular Compounds • If more than one atom, name the first element with a numerical prefix. • Name the second element with a numerical prefix and a suffix –ide. • For prefixes, drop o or a if the element name begins with a vowel. (Examples are monoxide and pentoxide. ) Examples: • N 2 O 4 dinitrogen tetroxide • OF 2 oxygen difluoride Mullis 2
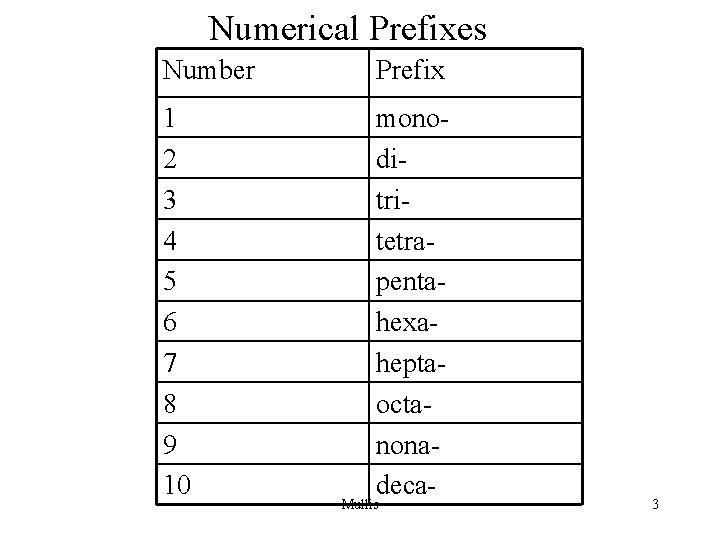
Numerical Prefixes Number Prefix 1 2 3 4 5 6 7 8 9 10 monoditritetrapentahexaheptaoctanonadeca- Mullis 3
Polyatomic ions • Poly = many • Atomic = atoms • Entire group of atoms is an ion with a positive or negative charge. • Within the polyatomic ion, atoms are bound covalently. • Examples: Carbonate ion CO 3 2 - Sulfate ion SO 4 2 S C Mullis 4
Chemical Equations • Law of conservation of mass: Atoms are neither created nor destroyed (in ordinary chemical reactions). –number of atoms on left = number of atoms on right • The correct formula must be shown for all reactants and products. –Use oxidation states and ionic charges to correctly write a formula –In balancing, do not split up molecules or change the formula. –Remember common polyatomic ions and diatomic molecules. Mullis 5
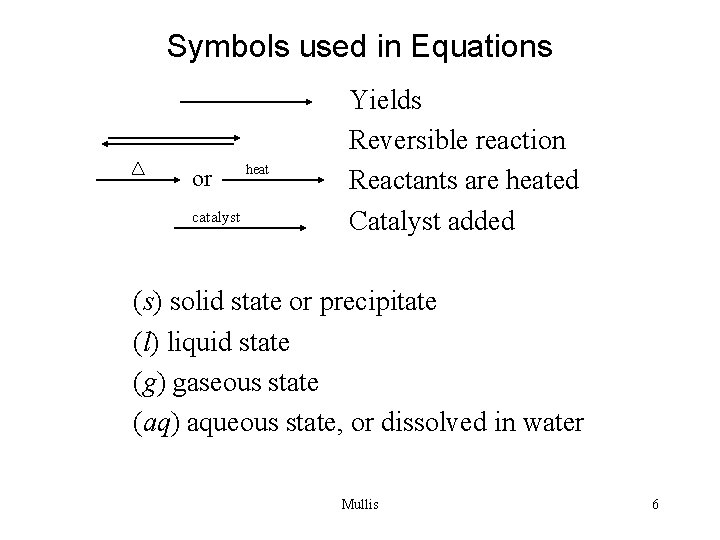
Symbols used in Equations or catalyst heat Yields Reversible reaction Reactants are heated Catalyst added (s) solid state or precipitate (l) liquid state (g) gaseous state (aq) aqueous state, or dissolved in water Mullis 6

Order for Balancing • MINOH method (Me know chemistry, said Tarzan as he climbed the stoichiome-tree. ) M - metals I - ions N – nonmetals O – oxygen * H – hydrogen Balance metals first. Balance polyatomic ions. Balance Cl, S, N. *An odd # on one side and even on other will require you to multiply the odd side to make it even! Mullis 7
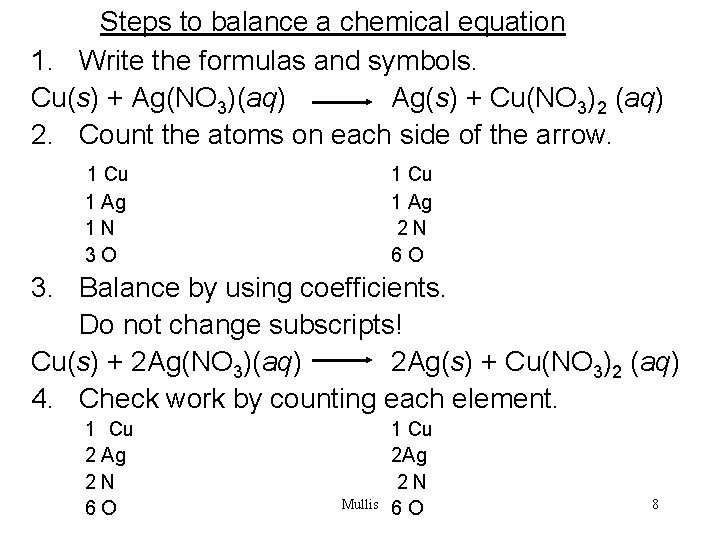
Steps to balance a chemical equation 1. Write the formulas and symbols. Cu(s) + Ag(NO 3)(aq) Ag(s) + Cu(NO 3)2 (aq) 2. Count the atoms on each side of the arrow. 1 Cu 1 Ag 1 N 3 O 1 Cu 1 Ag 2 N 6 O 3. Balance by using coefficients. Do not change subscripts! Cu(s) + 2 Ag(NO 3)(aq) 2 Ag(s) + Cu(NO 3)2 (aq) 4. Check work by counting each element. 1 Cu 2 Ag 2 N 6 O Mullis 1 Cu 2 Ag 2 N 6 O 8

Two Important Principles 1. Every chemical compound has a formula that cannot be altered. 2. A chemical reaction must account for every atom used. (Law of Conservation of Matter) Mullis 9
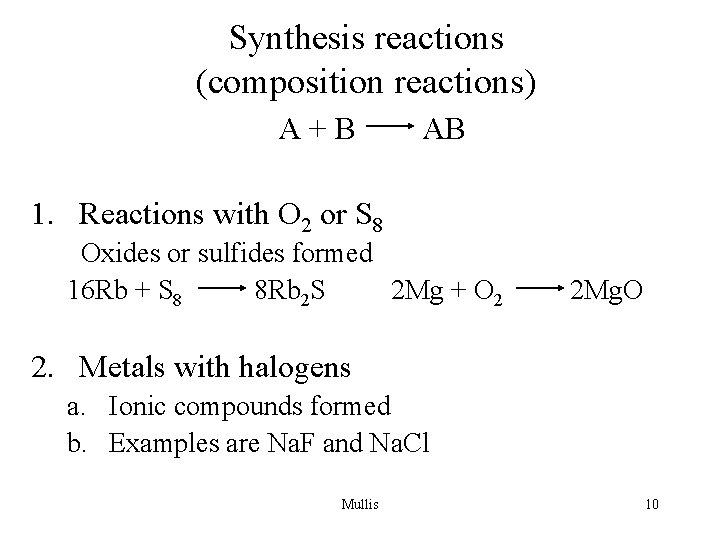
Synthesis reactions (composition reactions) A+B AB 1. Reactions with O 2 or S 8 Oxides or sulfides formed 16 Rb + S 8 8 Rb 2 S 2 Mg + O 2 2 Mg. O 2. Metals with halogens a. Ionic compounds formed b. Examples are Na. F and Na. Cl Mullis 10
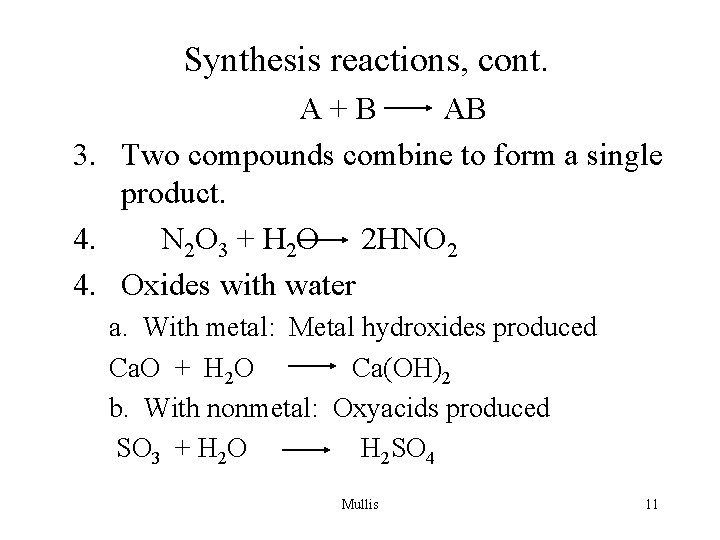
Synthesis reactions, cont. A+B AB 3. Two compounds combine to form a single product. 4. N 2 O 3 + H 2 O 2 HNO 2 4. Oxides with water a. With metal: Metal hydroxides produced Ca. O + H 2 O Ca(OH)2 b. With nonmetal: Oxyacids produced SO 3 + H 2 O H 2 SO 4 Mullis 11
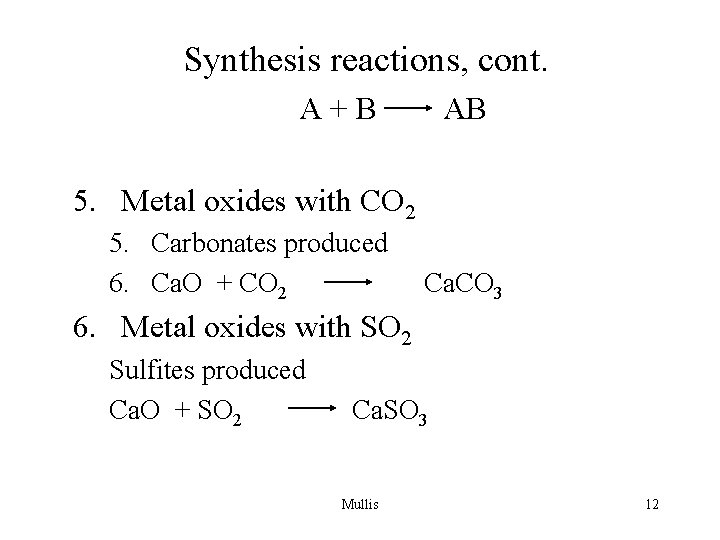
Synthesis reactions, cont. A+B AB 5. Metal oxides with CO 2 5. Carbonates produced 6. Ca. O + CO 2 Ca. CO 3 6. Metal oxides with SO 2 Sulfites produced Ca. O + SO 2 Ca. SO 3 Mullis 12
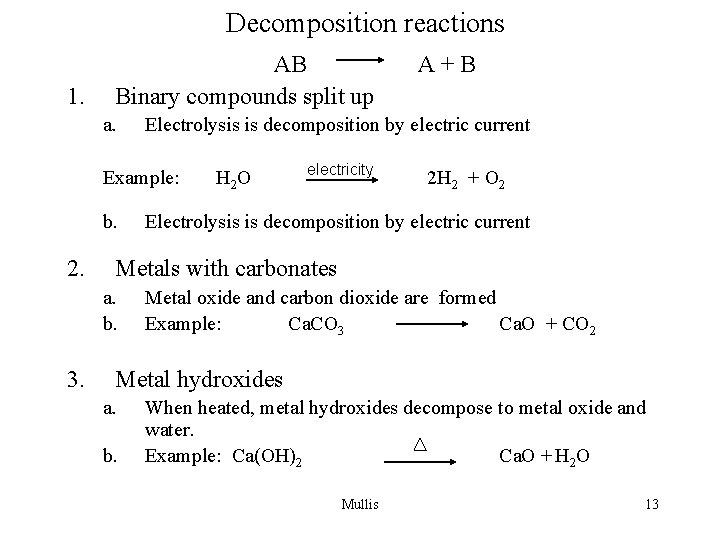
Decomposition reactions 1. AB Binary compounds split up a. Electrolysis is decomposition by electric current Example: b. 2. H 2 O electricity 2 H 2 + O 2 Electrolysis is decomposition by electric current Metals with carbonates a. b. 3. A+B Metal oxide and carbon dioxide are formed Example: Ca. CO 3 Ca. O + CO 2 Metal hydroxides a. b. When heated, metal hydroxides decompose to metal oxide and water. Example: Ca(OH)2 Ca. O + H 2 O Mullis 13
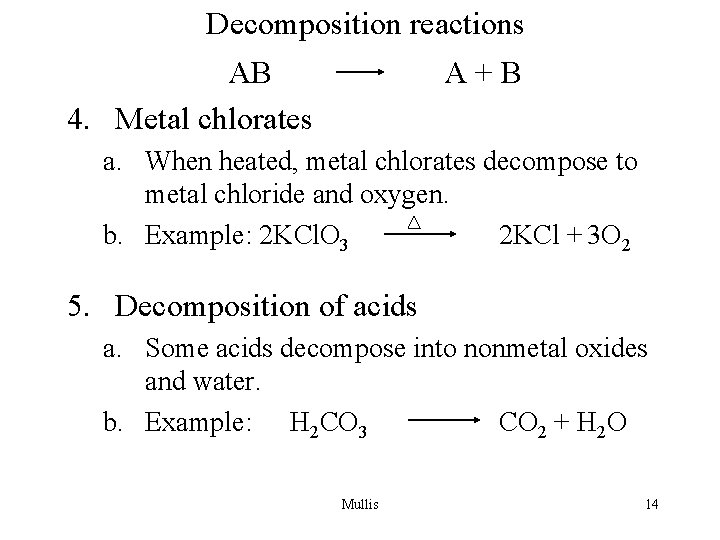
Decomposition reactions AB 4. Metal chlorates A+B a. When heated, metal chlorates decompose to metal chloride and oxygen. b. Example: 2 KCl. O 3 2 KCl + 3 O 2 5. Decomposition of acids a. Some acids decompose into nonmetal oxides and water. b. Example: H 2 CO 3 CO 2 + H 2 O Mullis 14
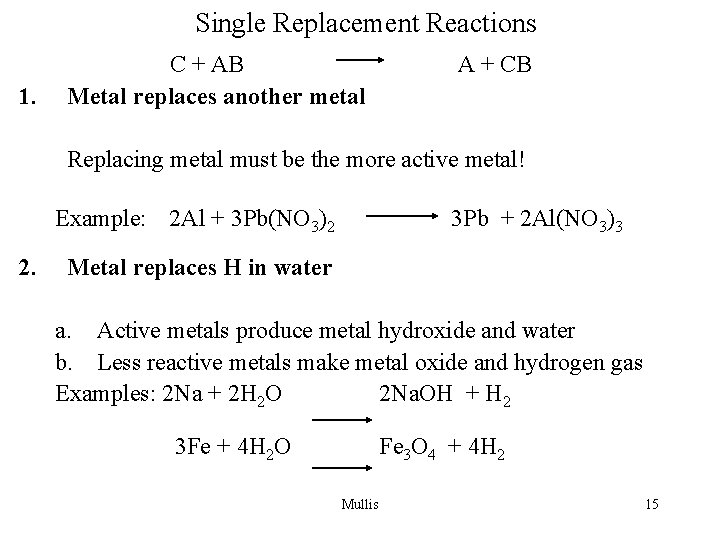
Single Replacement Reactions 1. C + AB Metal replaces another metal A + CB Replacing metal must be the more active metal! Example: 2 Al + 3 Pb(NO 3)2 2. 3 Pb + 2 Al(NO 3)3 Metal replaces H in water a. Active metals produce metal hydroxide and water b. Less reactive metals make metal oxide and hydrogen gas Examples: 2 Na + 2 H 2 O 2 Na. OH + H 2 3 Fe + 4 H 2 O Fe 3 O 4 + 4 H 2 Mullis 15
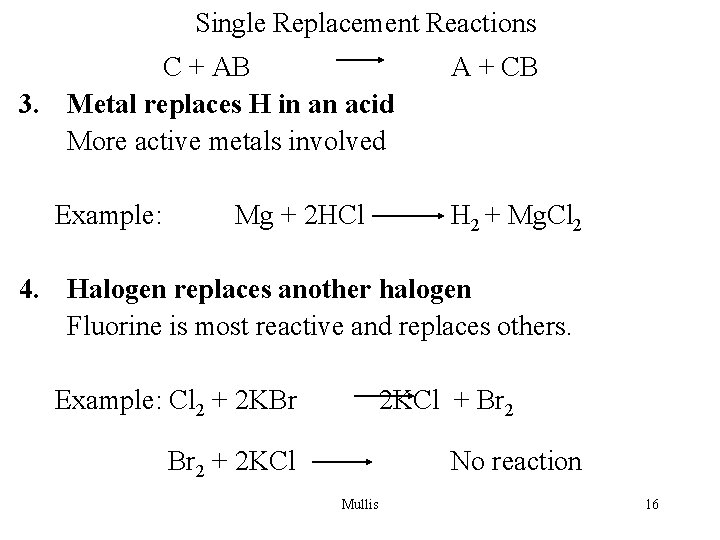
Single Replacement Reactions C + AB 3. Metal replaces H in an acid More active metals involved Example: Mg + 2 HCl A + CB H 2 + Mg. Cl 2 4. Halogen replaces another halogen Fluorine is most reactive and replaces others. Example: Cl 2 + 2 KBr 2 KCl + Br 2 + 2 KCl No reaction Mullis 16
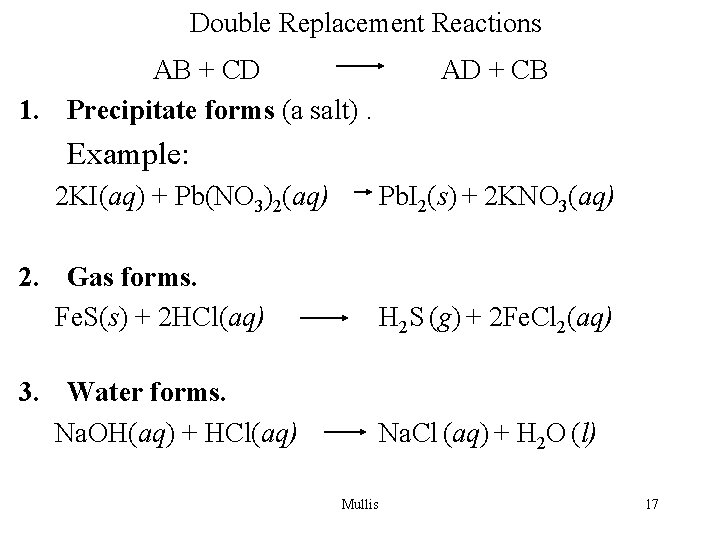
Double Replacement Reactions AB + CD 1. Precipitate forms (a salt). AD + CB Example: 2 KI(aq) + Pb(NO 3)2(aq) Pb. I 2(s) + 2 KNO 3(aq) 2. Gas forms. Fe. S(s) + 2 HCl(aq) H 2 S (g) + 2 Fe. Cl 2(aq) 3. Water forms. Na. OH(aq) + HCl(aq) Na. Cl (aq) + H 2 O (l) Mullis 17

Combustion Reactions CH 4 + 2 O 2 2 H 2 O + CO 2 • One of the reactants is oxygen. • In this class, all or our combustion reactions will be oxygen and hydrocarbons. • The products of this type of combustion reaction will always be water and carbon dioxide. • To quickly balance combustion reactions, START WITH WATER and multiply it by an EVEN coefficient. Mullis 18
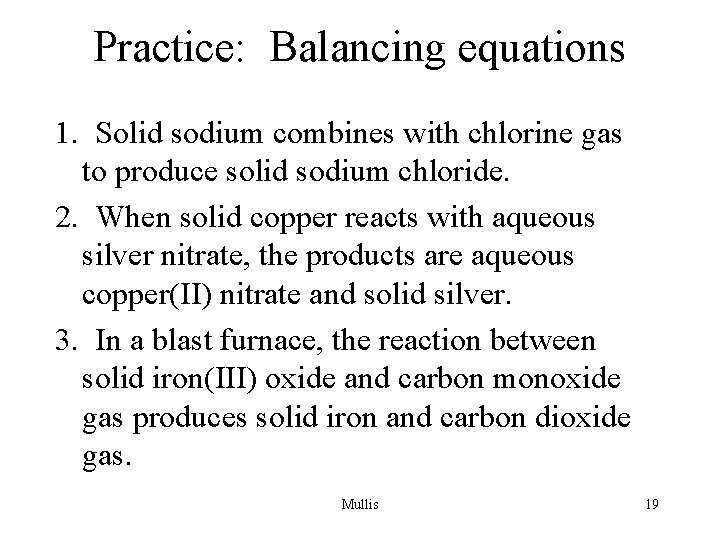
Practice: Balancing equations 1. Solid sodium combines with chlorine gas to produce solid sodium chloride. 2. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and solid silver. 3. In a blast furnace, the reaction between solid iron(III) oxide and carbon monoxide gas produces solid iron and carbon dioxide gas. Mullis 19
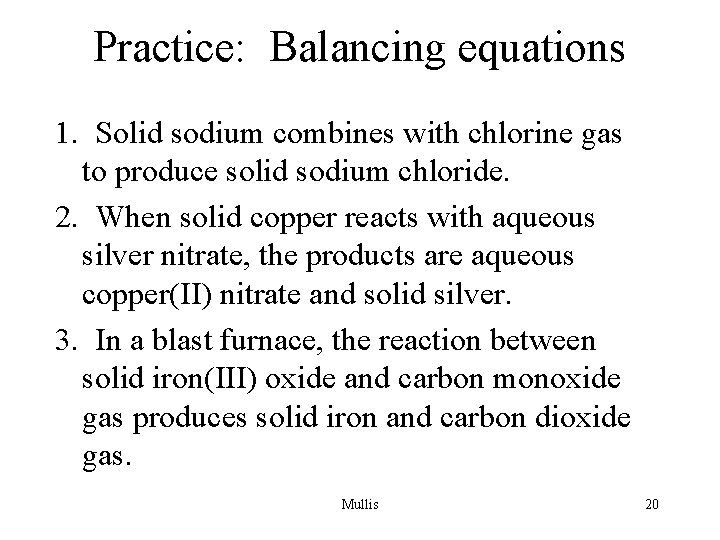
Practice: Balancing equations 1. Solid sodium combines with chlorine gas to produce solid sodium chloride. 2. When solid copper reacts with aqueous silver nitrate, the products are aqueous copper(II) nitrate and solid silver. 3. In a blast furnace, the reaction between solid iron(III) oxide and carbon monoxide gas produces solid iron and carbon dioxide gas. Mullis 20
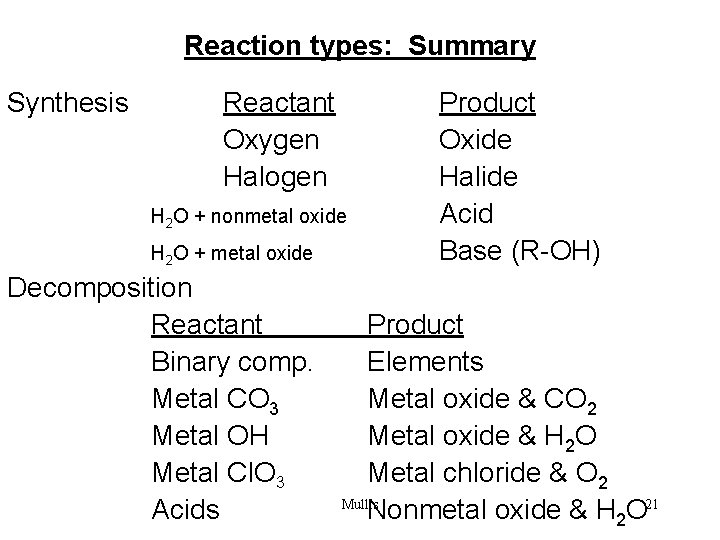
Reaction types: Summary Synthesis Reactant Oxygen Halogen H 2 O + nonmetal oxide H 2 O + metal oxide Decomposition Reactant Binary comp. Metal CO 3 Metal OH Metal Cl. O 3 Acids Product Oxide Halide Acid Base (R-OH) Product Elements Metal oxide & CO 2 Metal oxide & H 2 O Metal chloride & O 2 Mullis Nonmetal oxide & H 2 O 21
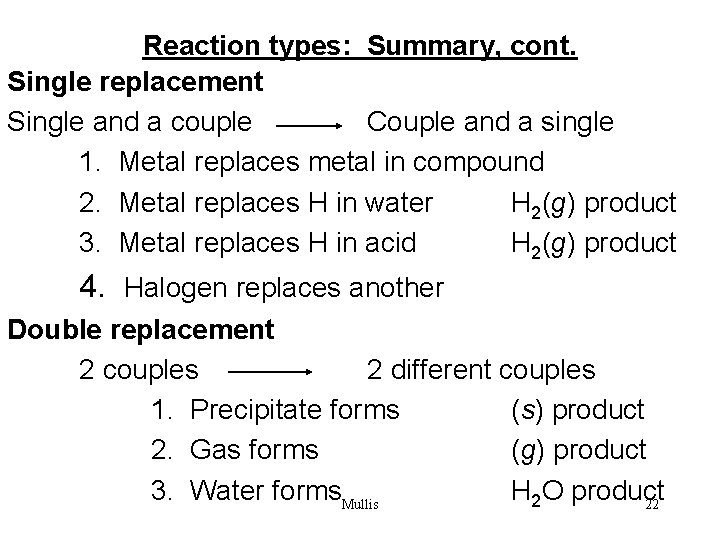
Reaction types: Summary, cont. Single replacement Single and a couple Couple and a single 1. Metal replaces metal in compound 2. Metal replaces H in water H 2(g) product 3. Metal replaces H in acid H 2(g) product 4. Halogen replaces another Double replacement 2 couples 2 different couples 1. Precipitate forms (s) product 2. Gas forms (g) product 3. Water forms. Mullis H 2 O product 22
- Slides: 22