Naming Compounds and Chemical Formulas Bonding Octet Rule

Naming Compounds and Chemical Formulas
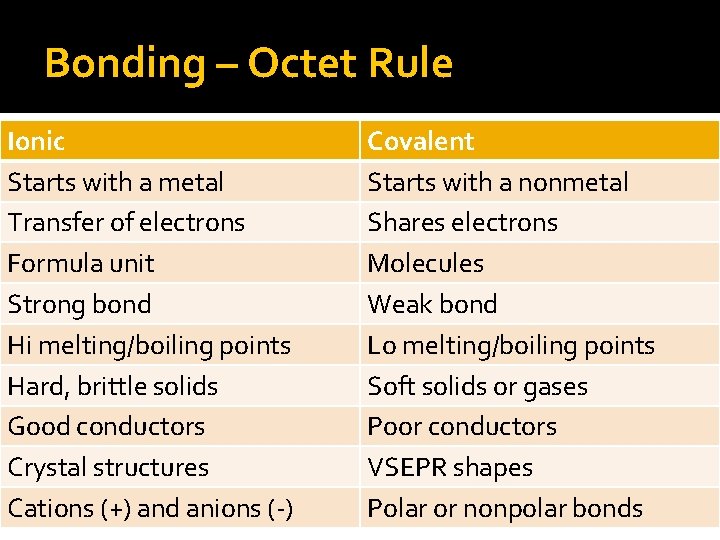
Bonding – Octet Rule Ionic Starts with a metal Transfer of electrons Formula unit Strong bond Hi melting/boiling points Hard, brittle solids Good conductors Crystal structures Cations (+) and anions (-) Covalent Starts with a nonmetal Shares electrons Molecules Weak bond Lo melting/boiling points Soft solids or gases Poor conductors VSEPR shapes Polar or nonpolar bonds
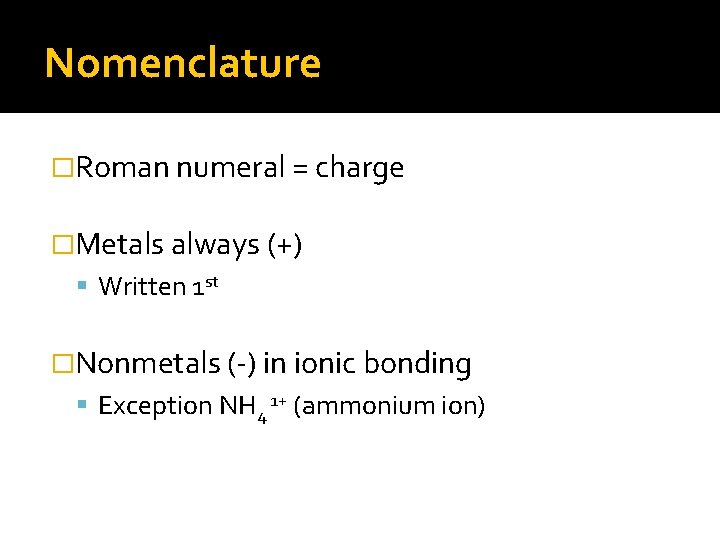
Nomenclature �Roman numeral = charge �Metals always (+) Written 1 st �Nonmetals (-) in ionic bonding Exception NH 4 1+ (ammonium ion)

Endings �ide = original element (nonmetal) �ate and ite = polyatomic ions 2 exceptions: CN 1 - (cyanide) and OH 1 - (hydroxide) Don’t confuse valence # with charge. Valence # is the number of electrons in the outer shell Charge is the transfer of electrons
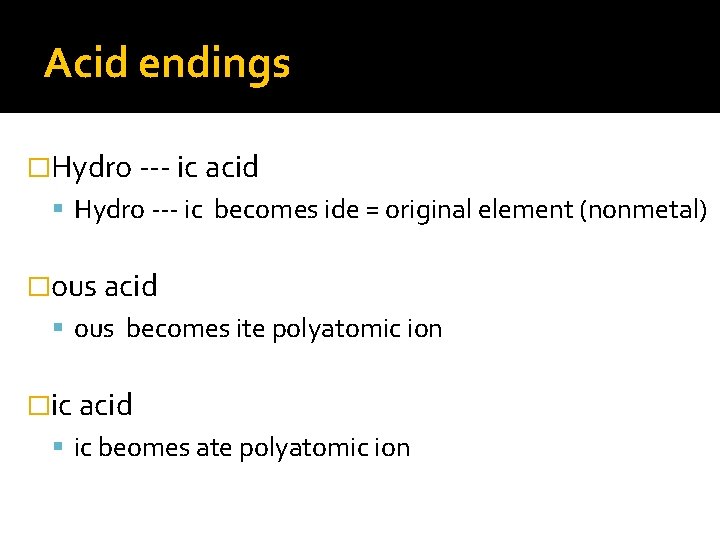
Acid endings �Hydro --- ic acid Hydro --- ic becomes ide = original element (nonmetal) �ous acid ous becomes ite polyatomic ion �ic acid ic beomes ate polyatomic ion
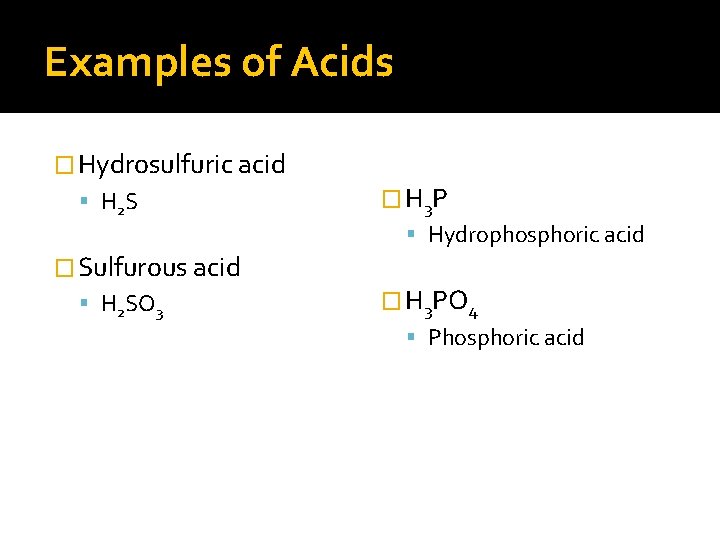
Examples of Acids � Hydrosulfuric acid H 2 S � H 3 P Hydrophosphoric acid � Sulfurous acid H 2 SO 3 � H 3 PO 4 Phosphoric acid
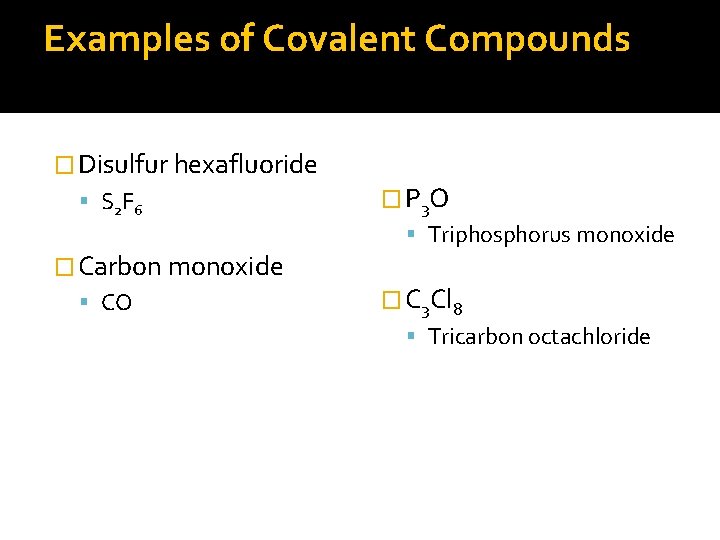
Examples of Covalent Compounds � Disulfur hexafluoride S 2 F 6 � Carbon monoxide CO � P 3 O Triphosphorus monoxide � C 3 Cl 8 Tricarbon octachloride
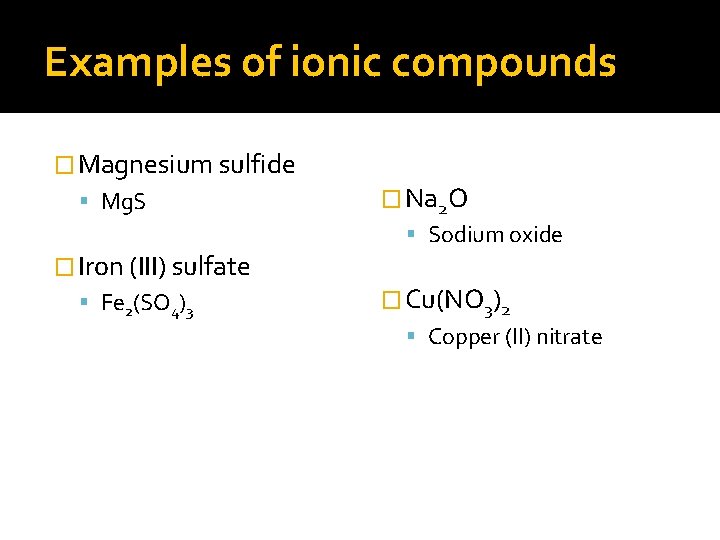
Examples of ionic compounds � Magnesium sulfide Mg. S � Iron (III) sulfate Fe 2(SO 4)3 � Na 2 O Sodium oxide � Cu(NO 3)2 Copper (II) nitrate
- Slides: 8