Naming Common Compounds Classifying Inorganic Compounds There are




















- Slides: 29

Naming Common Compounds

Classifying Inorganic Compounds There are three types of common inorganic compounds. They are • Ionic compounds • Molecular compounds • Acids

Ionic Compounds • Ionic compounds are composed of positively and negatively charged ions and are held together by electrostatic attractions called ionic bonds. • Ionic compounds do not contain molecules

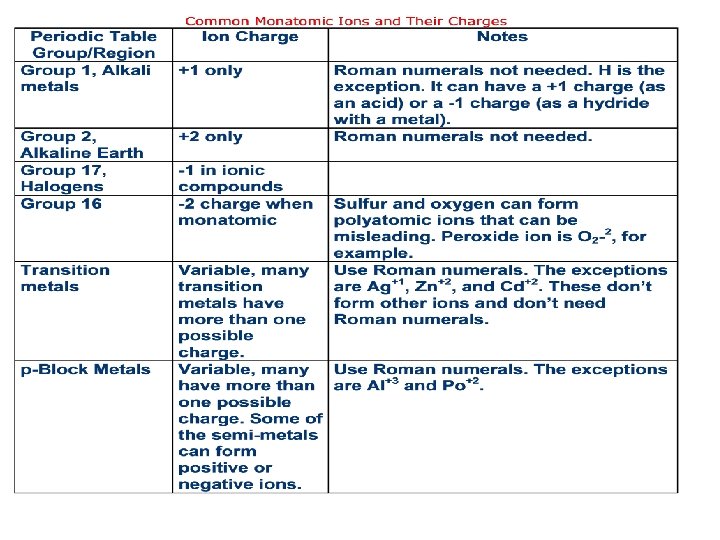
Identifying Ionic Compounds • Metals tend to form positive ions, and non-metals tend to form negative ions. • Hydrogen, H+1, and ammonium ion, NH 4+1, are the only common positive ions that do not contain a metal. • Of these two, ammonium is found in ionic compounds, and hydrogen is found in acids. • To identify a compound as being ionic, scan the name or the formula and identify the elements. • If the compound contains a metal (or the ammonium ion) and at least one non-metal, it is almost always an ionic compound.

Naming Ionic Compounds • Ionic compound names have two parts: the cation (positive ion) name and the anion (negative ion) name. • The positive ion is written first. Example: K 2 S is potassium sulfide. Potassium is the name of the cation; sulfide is the name of the anion. • Cations have the same name as their element, though they may also have a number. Example: Mg+2 is the magnesium ion; Fe+3 is the iron (III) ion. • Anions with only one atom have the same root name as their element but end with the suffix “-ide. ” Example: Cl-1 is the chloride ion. • You will need to learn the names of the monatomic (single atom) ions and their charges. This is a periodic property. • Many metals can form more than one cation. These metals can be found in the transition and lower p-block parts of the periodic table. • The names of these ions have Roman Numerals in parenthesis after the metal name. This number is the charge of the ion. Example: Fe. Cl 3 is iron (III) chloride, because the iron in this compound has a +3 charge. Fe. Cl 2 is iron (II) chloride, because the iron ion has a +2 charge.


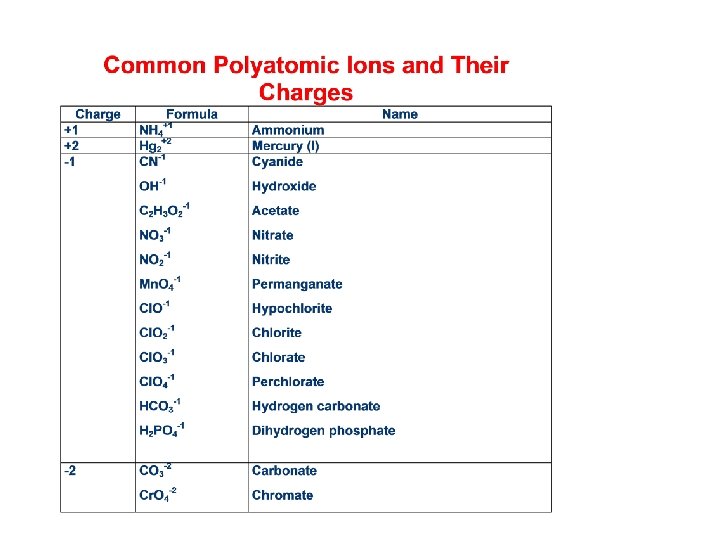
Ionic Compounds having Polyatomic Ions • Many ionic compounds have polyatomic (many atom) ions. • To name these, name the cation the anion. Example: NH 4 OH is ammonium hydroxide. • In order to name ionic compounds (and acids) you will need to memorize the names of the common polyatomic ions.




Formulas of Ionic Compounds • If given the name, identify the formulas of each of the ions then use subscripts to make the charges neutral. Examples: • Magnesium bromide contains the ions Mg+2 and Br -1, so the formula is Mg. Br 2. • Tin (IV) sulfate contains the ions Sn+4 and SO 4 -2, so the formula is Sn(SO 4)2.



Acids • Acids are substances that react with water to form an aqueous hydrogen ion, H+1(aq). • These compounds have characteristics in common with both ionic and molecular compounds • Identifying Acids • There are different systems for classifying acids. Some acids have names that fit the following rules. Others have names that fit the ionic or binary molecular compound naming rules. • To determine if a compound is an acid for naming purposes, scan the chemical formula. The formula for an acid begins with H.

Naming Acids • Scan the formula. Identify the anion. Acid names use the name of the anion as a root word for the acid name. Example: HF is hydrofluoric acid. It has a root word “fluor-” from the name of the fluoride ion. • If the name of the anion ends with the suffix “-ate” the acid name will have the format “___ic acid. ” Example: H 2 SO 4 is sulfur ic acid because SO 4 -2 is the sulfate ion. • If the name of the anion ends in with the suffix “_ite” the acid name will have the format “___ous acid. ” Example: HNO 2 is nitrous acid because NO 2 -1 is the nitrite ion. • If the name of the anion ends with the suffix “-ide” the acid name will have the format “hydro___ic acid. ” Example: HCN is hydrocyanic acid because CN-1 is the cyanide ion.






Molecular Compounds • Molecular compounds are composed of molecules in which atoms share electrons via bonds. • Identifying Molecular Compounds • Molecular compounds contain two or more non-metals but not the ammonium ion, NH 4+1. • To determine if a compound is molecular scan the formula for a metal or the ammonium ion—these will be written at the beginning of the formula. If the compound does not contain a metal, it is probably molecular. • Compound that have formulas beginning with H and anion with names ending in “-ide” can be named as acids or molecular compounds. • If the compound is in aqueous solution, name it as an acid. Example: HF(aq) is hydrofluoric acid. • If the compound is a gas, name it as a molecular compound. Example: H 2 S(g) is hydrogen sulfide. • Organic compounds contain carbon. Some organic compounds follow the general rules for binary molecular compounds (carbon dioxide, carbon monoxide, carbon tetrachloride) but most follow a special naming system.

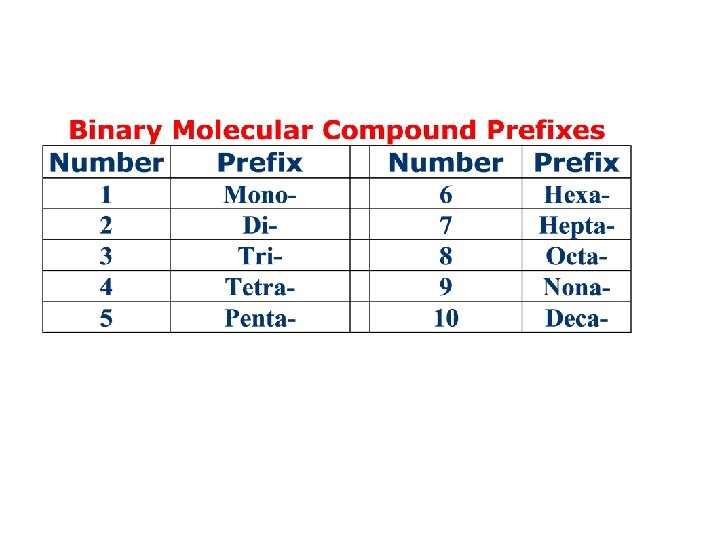
Molecular Compounds Binary Molecular Compounds • Binary molecular compounds contain two nonmetals. • Some have common names from antiquity. Common names you will need to know: ammonia, NH 3, and water, H 2 O. Naming Binary Molecular Compounds • The first element in the formula is the first element in the name. Example: CO 2 is carbon dioxide; the same order in the name as in the formula. • Use prefixes to indicate the number of atoms of each of the elements in the molecule. Example: CO 2 is carbon dioxide; di- prefix for two oxygen atoms.


Molecular Compounds • The two elements in the compound are named as two separate words. Each word will have its own prefix. The prefix “mono-” is not used for the first element. Example: CO 2 is carbon dioxide NOT monocarbon dioxide. • End the name with the suffix “-ide. ” Example: CO 2 is carbon dioxide; the name ends with “-ide. ” • More Examples • SF 6 is sulfur hexafluoride. • N 2 O 5 is dinitrogen pentoxide. • Cl 2 O is dichlorine monoxide. • Note that an extra vowel was dropped in the names of the last two. This makes the names easier to pronounce.



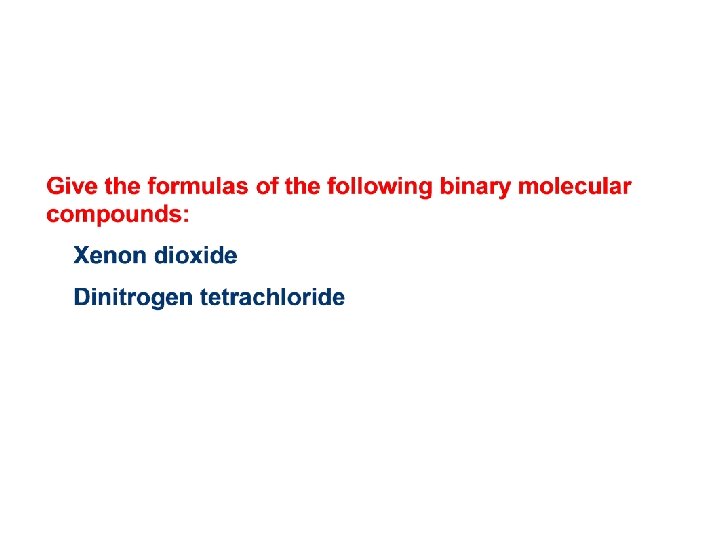
Formulas of Binary Molecular Compounds • To write the formula from the name of a binary molecular compound, scan the name and identify the two non-metallic elements. • Write the symbol for the first element first and the second element second. • Use the numbers from the prefixes as subscripts. Example: • Tetraphosphorus decasulfide has P and S as the elements in its formula. • P will appear first in the formula. • The subscript on P is 4. The subscript on S is 10. • The formula is P 4 S 10.

