Naming Binary Molecular Compounds Binary Molecular Compounds Compounds

Naming Binary Molecular Compounds
Binary Molecular Compounds § Compounds between two nonmetals § First element in the formula is named first. § Keeps its element name § Gets a prefix if there is a subscript on it § Second element is named second § Use the root of the element name plus the -ide suffix § Always use a prefix on the second element

§ 1 = mon(o) § 2 = di § 3 = tri § 4 = tetra § 5 = penta § 6 = hexa § 7 = hepta § 8 = octa § 9 = nona § 10 = deka List of Prefixes
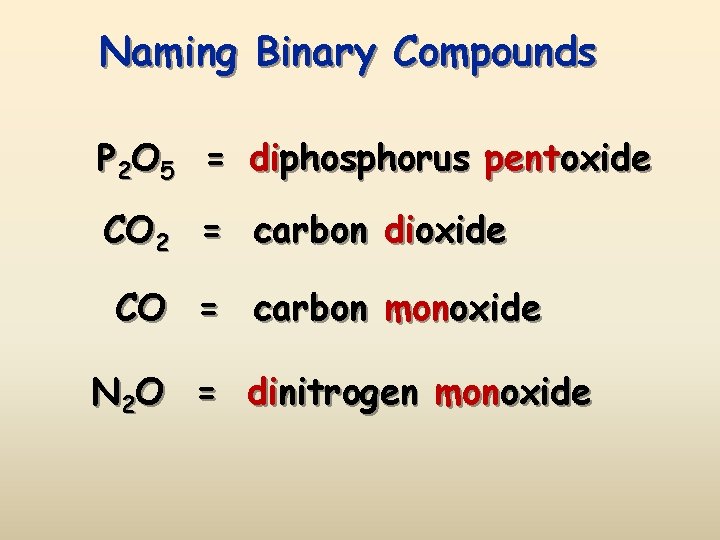
Naming Binary Compounds P 2 O 5 = diphosphorus pentoxide CO 2 = carbon dioxide CO = carbon monoxide N 2 O = dinitrogen monoxide

Practice – Write the Formula Compound Name Compound Formula Carbon dioxide Carbon monoxide Diphosphorus pentoxide Dinitrogen monoxide Silicon dioxide Carbon tetrabromide Sulfur dioxide Phosphorus pentabromide Iodine trichloride Nitrogen triiodide Dinitrogen trioxide Check next slide for answers

Answers – Write the Formula Compound Name Compound Formula Carbon dioxide CO 2 Carbon monoxide CO Diphosphorus pentoxide P 2 O 5 Dinitrogen monoxide N 2 O Silicon dioxide Si. O 2 Carbon tetrabromide CBr 4 Sulfur dioxide SO 2 Phosphorus pentabromide PBr 5 Iodine trichloride ICl 3 Nitrogen triiodide NI 3 Dinitrogen trioxide N 2 O 3
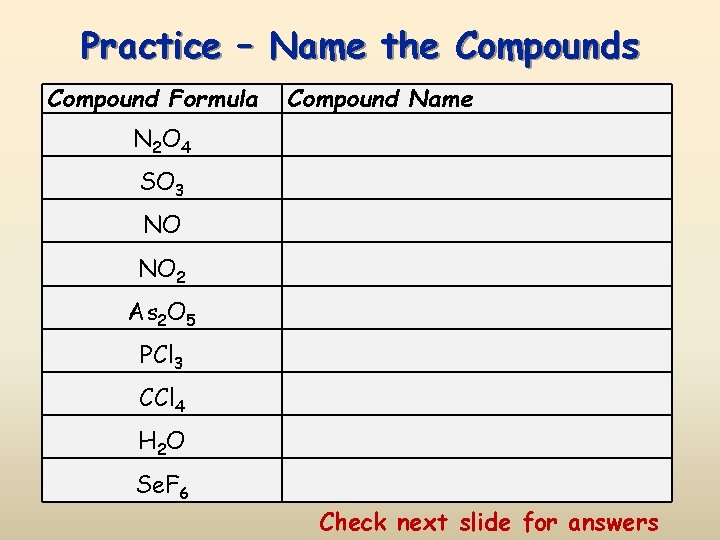
Practice – Name the Compounds Compound Formula Compound Name N 2 O 4 SO 3 NO NO 2 As 2 O 5 PCl 3 CCl 4 H 2 O Se. F 6 Check next slide for answers
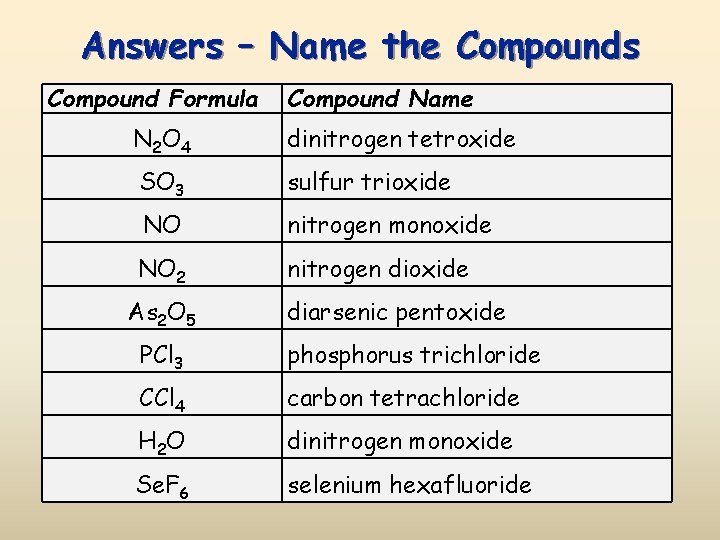
Answers – Name the Compounds Compound Formula Compound Name N 2 O 4 dinitrogen tetroxide SO 3 sulfur trioxide NO nitrogen monoxide NO 2 nitrogen dioxide As 2 O 5 diarsenic pentoxide PCl 3 phosphorus trichloride CCl 4 carbon tetrachloride H 2 O dinitrogen monoxide Se. F 6 selenium hexafluoride
- Slides: 8