Naming and writing formulas for Covalent bonds Unit

Naming and writing formulas for Covalent bonds Unit 5 Notes #4

• Reminder: Binary molecular compounds are made of two nonmetals • The name identifies the elements in the formula and must indicate the number of each atom of each element • Name the elements in order listed in the formula • Use prefixes to indicate the number of each kind of atom

Prefix Mono Di Tri Tetra Penta Hexa Hepta Octa Nona Deca Number 1 2 3 4 5 6 7 8 9 10

• Omit the prefix mono only when the formula contains one of the first element in the name • The first name takes the name found on the periodic table • The second name takes the name found on the PT, but ends in – ide

• NO 2 is named nitrogen dioxide (nitrogen is named the same as you see it on the PT and oxygen takes the –ide suffix. The “di” is used to indicate 2 oxygens, but the prefix “mono” is omitted b/c you don’t use “mono” when there is only on of the first element). Examples

• Cl 3 S 8 is named trichlorine octasulfide (The prefix “tri” is used to indicate there are 3 chlorines and the prefix “octa” is used to indicate 8 sulfurs. Chlorine keeps its name, while sulfur takes the -ide ending) Examples

• CO is named carbon monoxide (there is no “mono” needed to indicate only 1 carbon b/c carbon is the first element, but “mono” is needed to indicate there is only 1 oxygen b/c oxygen is the second element. Carbon keeps its name, while oxygen takes on the –ide ending) Examples

• Use the prefix to tell you the subscript of each element. • Then, Write the correct name for the two elements using the correct subscripts Writing formulas

Name Diphosphorous trioxide Iodine heptafluoride Nonoxide tribromide Tetraselenium mononitride Examples Formula
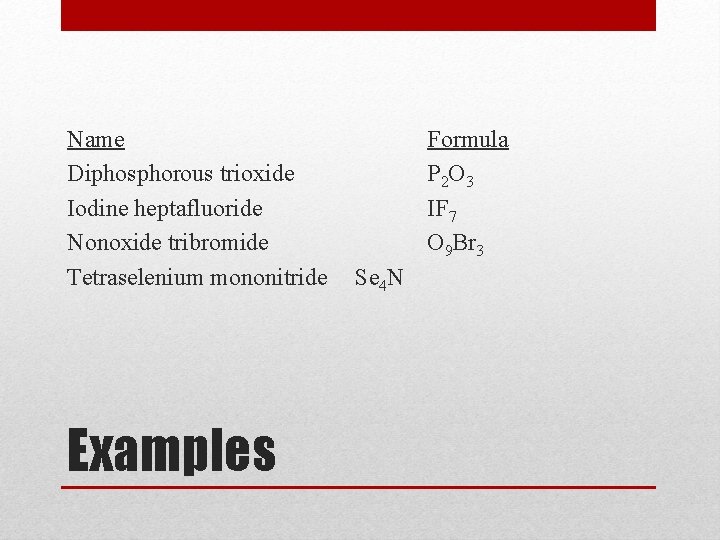
Name Diphosphorous trioxide Iodine heptafluoride Nonoxide tribromide Tetraselenium mononitride Examples Formula P 2 O 3 IF 7 O 9 Br 3 Se 4 N
- Slides: 10