Naming Acids and Bases Acids Arrhenius definition Break

Naming Acids and Bases
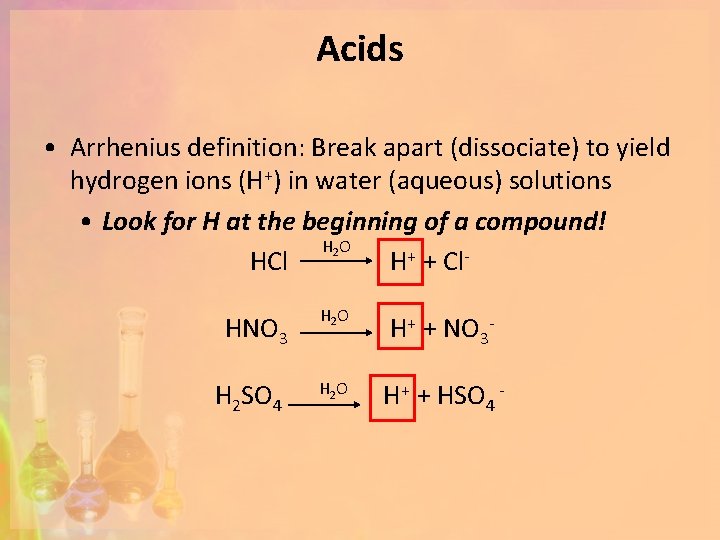
Acids • Arrhenius definition: Break apart (dissociate) to yield hydrogen ions (H+) in water (aqueous) solutions • Look for H at the beginning of a compound! H 2 O HCl H+ + Cl. HNO 3 H 2 SO 4 H 2 O H+ + NO 3 - H 2 O H+ + HSO 4 -

Naming Acids Anion Ending Acid Name -ide hydro-(stem)-ic acid -ite (stem)-ous acid -ate (stem)-ic acid Name or give the formula of the following acids: 1. H 2 SO 4 2. Oxalic acid 3. HF
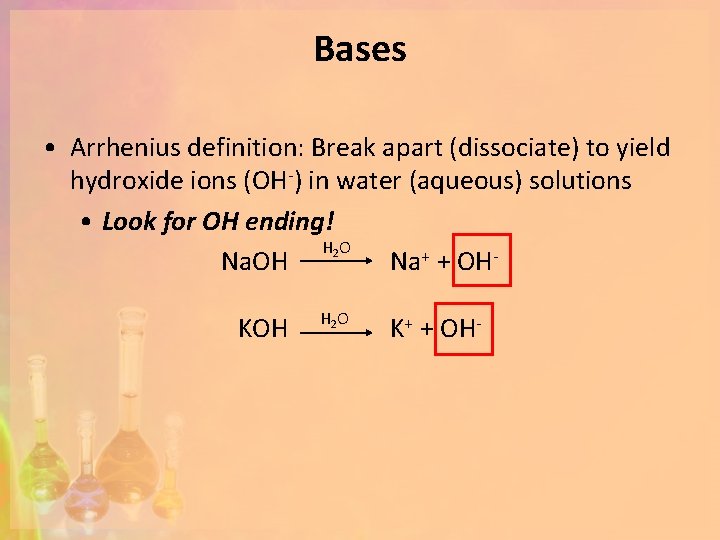
Bases • Arrhenius definition: Break apart (dissociate) to yield hydroxide ions (OH-) in water (aqueous) solutions • Look for OH ending! H 2 O Na. OH Na+ + OHKOH H 2 O K+ + OH-
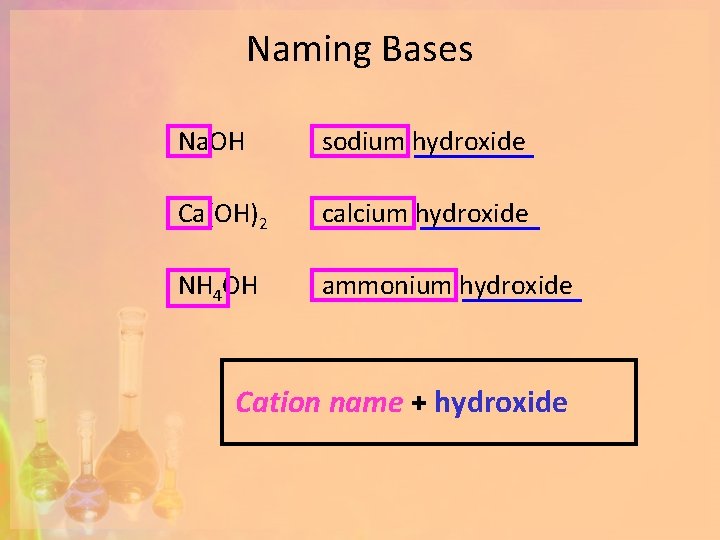
Naming Bases Na. OH sodium hydroxide Ca(OH)2 calcium hydroxide NH 4 OH ammonium hydroxide Cation name + hydroxide

Naming Bases Name the following bases: 1. Ba(OH)2 2. KOH 3. Al(OH)3 Cation name + hydroxide

More Naming Practice Name the following acids/bases or write their formula: 1. H 3 PO 4 2. Nitrous acid 3. Mg(OH)2 Anion Ending Acid Name 4. Hydrobromic acid -ide hydro-(stem)-ic acid 5. Lithium hydroxide -ite (stem)-ous acid 6. H 2 S -ate (stem)-ic acid
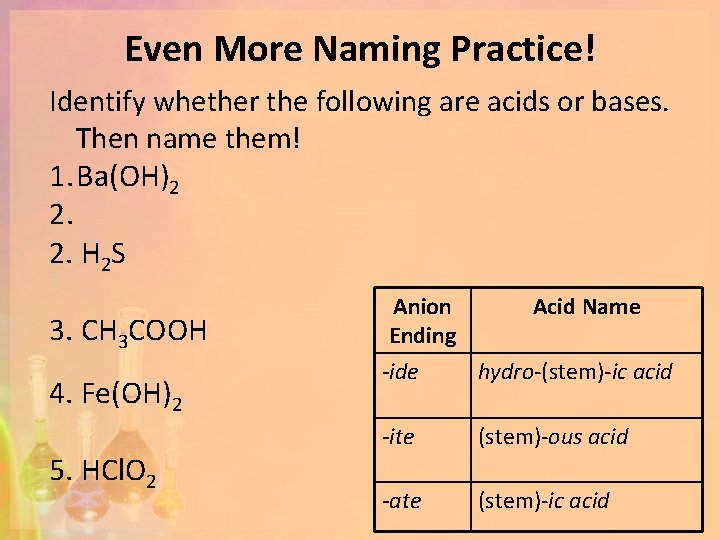
Even More Naming Practice! Identify whether the following are acids or bases. Then name them! 1. Ba(OH)2 2. 2. H 2 S 3. CH 3 COOH 4. Fe(OH)2 5. HCl. O 2 Anion Ending Acid Name -ide hydro-(stem)-ic acid -ite (stem)-ous acid -ate (stem)-ic acid
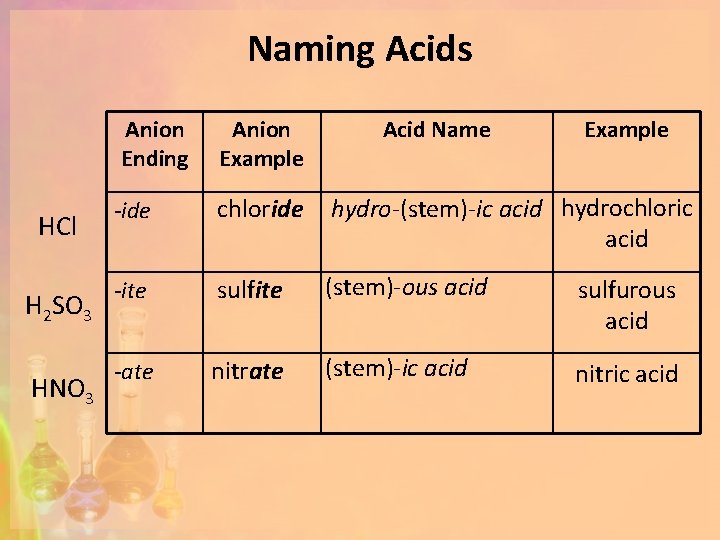
Naming Acids HCl H 2 SO 3 HNO 3 Anion Ending Anion Example Acid Name Example -ide chloride hydro-(stem)-ic acid hydrochloric acid -ite sulfite (stem)-ous acid sulfurous acid -ate nitrate (stem)-ic acid nitric acid
- Slides: 9