Naming Acids and Bases Acids and bases are

Naming Acids and Bases

Acids and bases are a form of ionic compound. Like all other ionic compounds, acids and bases break apart into their ions when they are dissolved in water. Acids and bases are often defined by the ions that they break into. HCl H+ + Cl - Important Fact: Acids and Bases are both corrosive!
Arrhenius Definition of Acids and Bases Svante Arrhenius was a scientist who defined acids and bases. This is the simplest definition of an acid and a base. He defined an acid as any substance which donates a hydrogen ion (H+). He defined a base as any substance which donates a hydroxide ion (OH-).
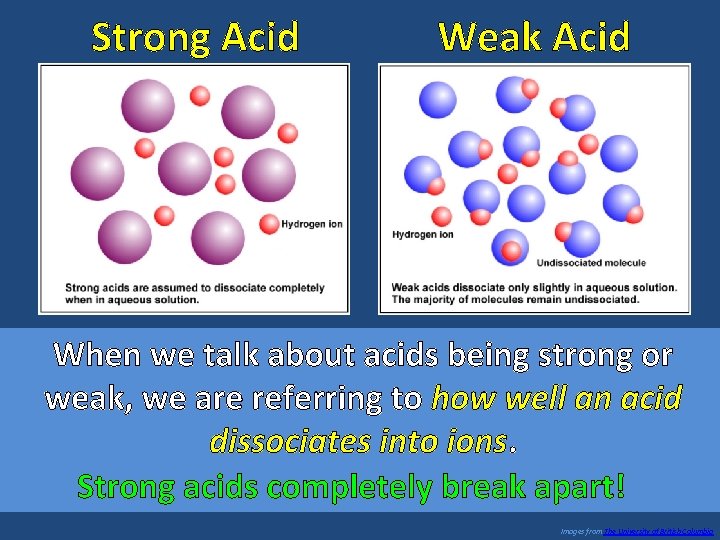
Strong Acid Weak Acid When we talk about acids being strong or weak, we are referring to how well an acid dissociates into ions. Strong acids completely break apart! Images from The University of British Columbia
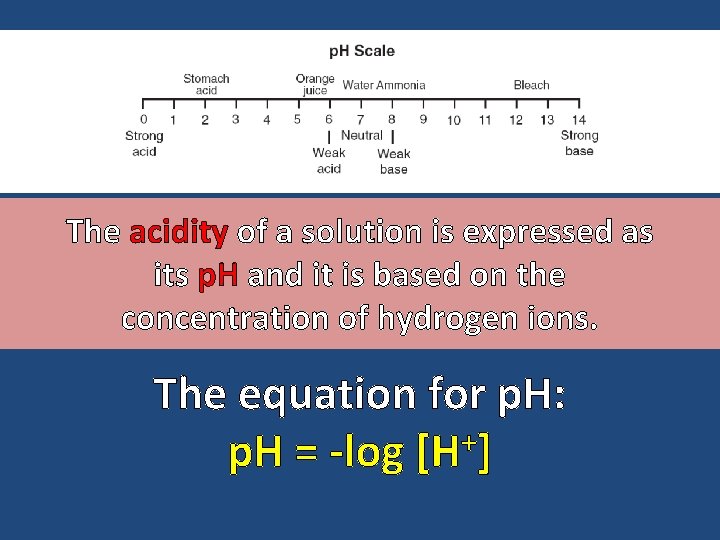
The acidity of a solution is expressed as its p. H and it is based on the concentration of hydrogen ions. The equation for p. H: + p. H = -log [H ]
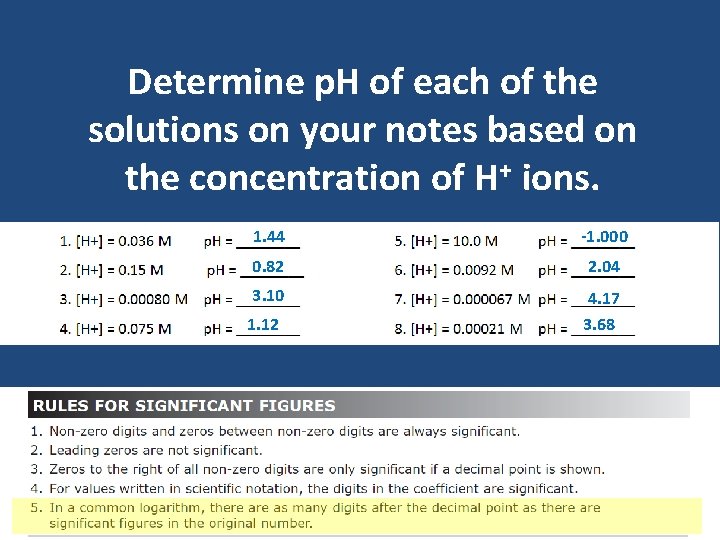
Determine p. H of each of the solutions on your notes based on the concentration of H+ ions. 1. 44 -1. 000 0. 82 2. 04 3. 10 4. 17 3. 68 1. 12
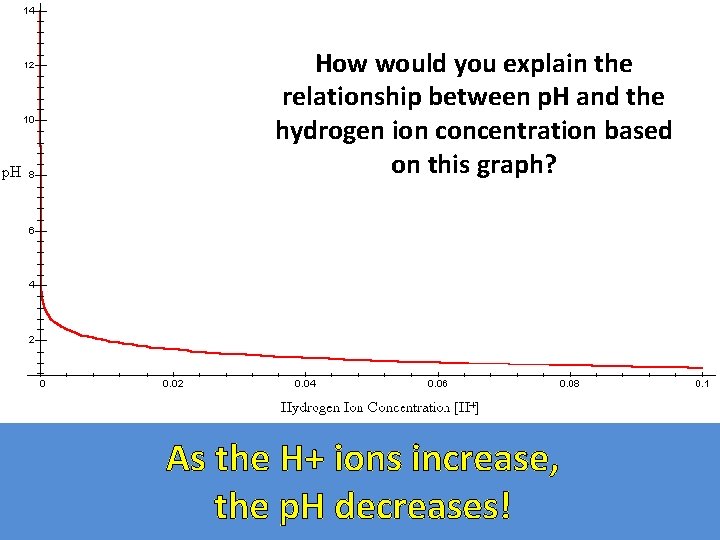
How would you explain the relationship between p. H and the hydrogen ion concentration based on this graph? As the H+ ions increase, the p. H decreases!
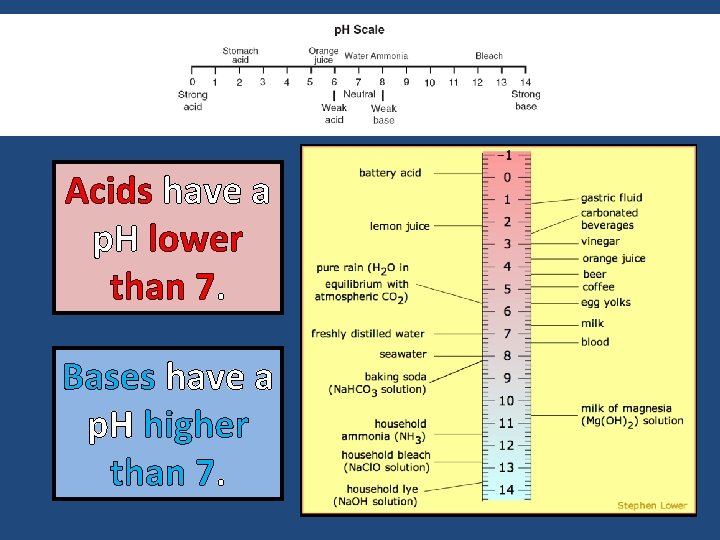
Acids have a p. H lower than 7. Bases have a p. H higher than 7.
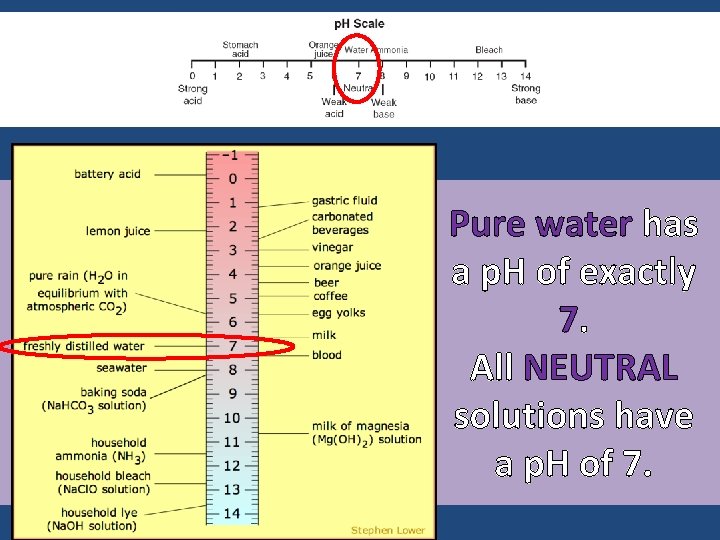
Pure water has a p. H of exactly 7. All NEUTRAL solutions have a p. H of 7.

The acidity of a solution can be precisely measured using a p. H meter.

It can be determined if a solution is acidic or basic by using litmus paper. Acids turn litmus paper RED. Bases turn litmus paper BLUE.

Naming Acids When it comes to naming acids, there are two categories you need to be familiar with: Binary Acids: These acids are made of H+ and one other element (often a halogen). Ex: HCl, HF, HBr, HI Acids of Oxyanions: These acids are made of H+ and a polyatomic ion containing oxygen. Ex: H 2 SO 4, HNO 3, H 3 PO 4, H 2 CO 3
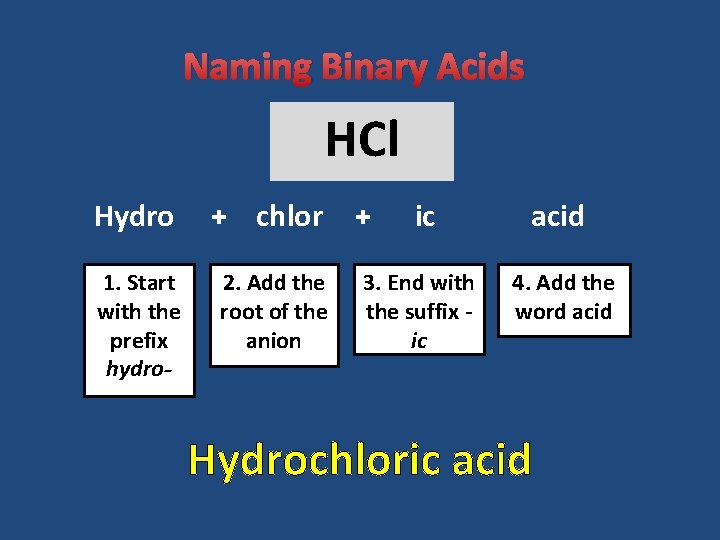
Naming Binary Acids HCl Hydro + chlor 1. Start with the prefix hydro- 2. Add the root of the anion + ic acid 3. End with the suffix ic 4. Add the word acid Hydrochloric acid
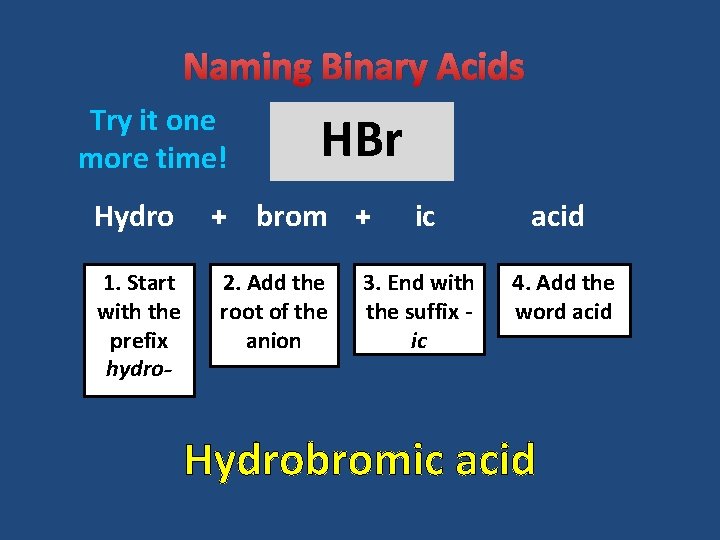
Naming Binary Acids Try it one more time! Hydro 1. Start with the prefix hydro- HBr + brom + 2. Add the root of the anion ic acid 3. End with the suffix ic 4. Add the word acid Hydrobromic acid

Naming Binary Acids It is very important that you remember that binary acids (HF, HCl, HBr, HI) always begin with the prefix hydro. This prefix will NOT appear in the names of acids made from oxyanions.
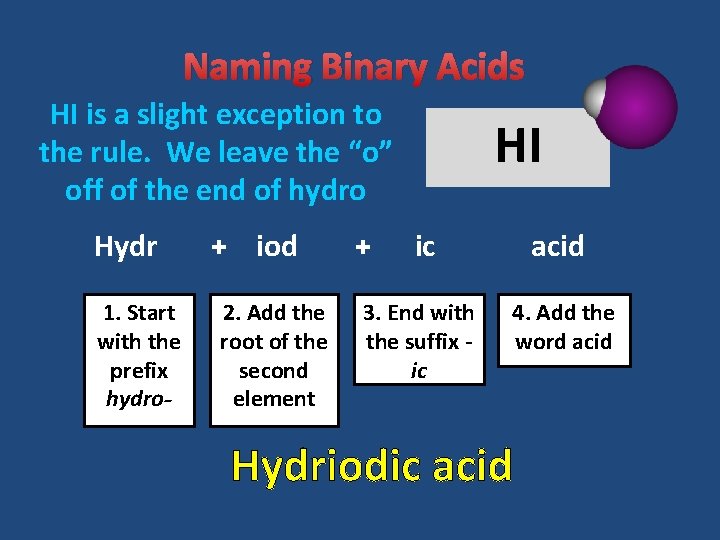
Naming Binary Acids HI is a slight exception to the rule. We leave the “o” off of the end of hydro Hydr 1. Start with the prefix hydro- + iod 2. Add the root of the second element + HI ic acid 3. End with the suffix ic 4. Add the word acid Hydriodic acid
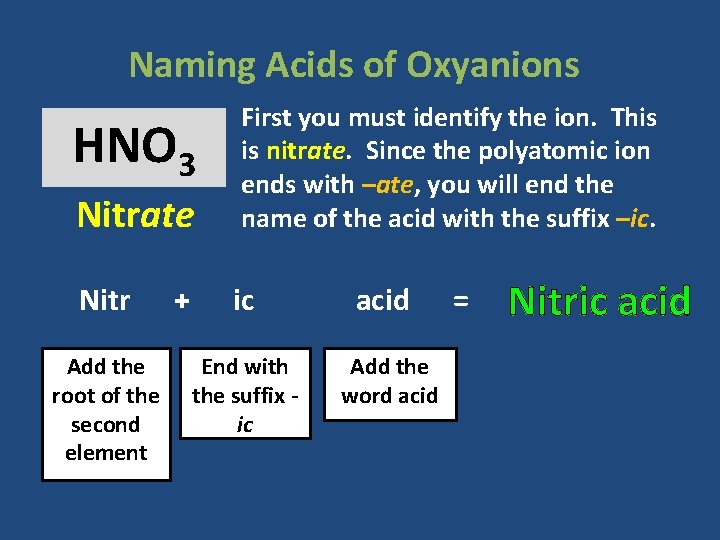
Naming Acids of Oxyanions Nitrate First you must identify the ion. This is nitrate. Since the polyatomic ion ends with –ate, you will end the name of the acid with the suffix –ic. Nitr ic acid End with the suffix ic Add the word acid HNO 3 Add the root of the second element + = Nitric acid
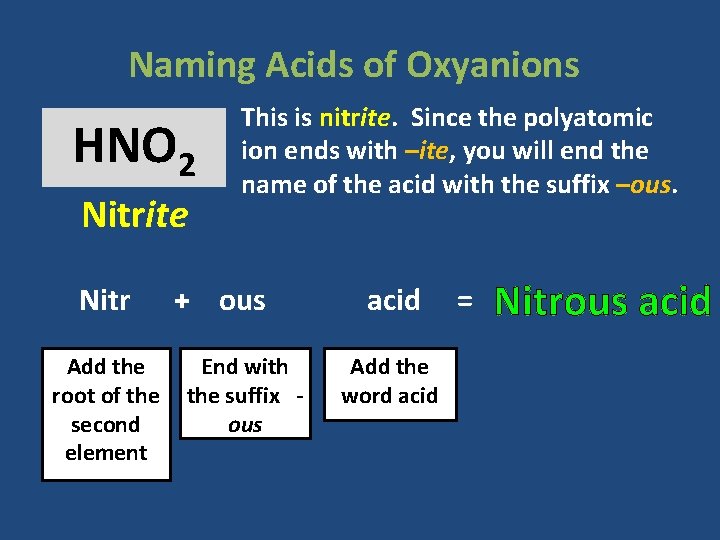
Naming Acids of Oxyanions HNO 2 Nitrite Nitr Add the root of the second element This is nitrite. Since the polyatomic ion ends with –ite, you will end the name of the acid with the suffix –ous. + ous End with the suffix ous acid Add the word acid = Nitrous acid
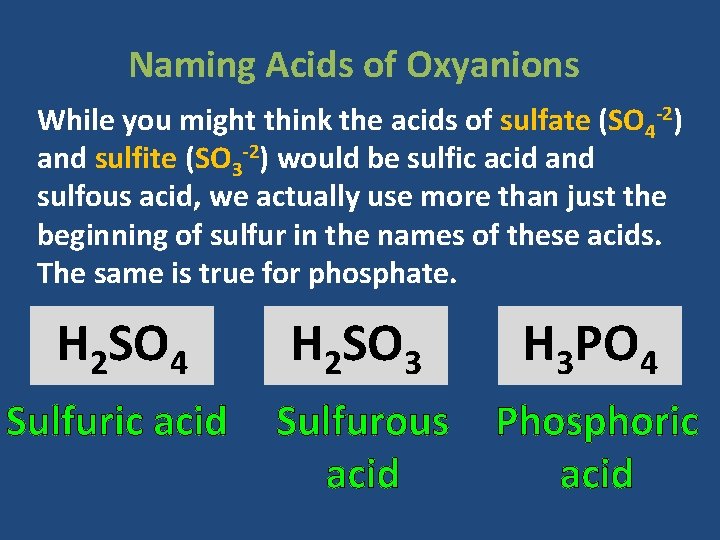
Naming Acids of Oxyanions While you might think the acids of sulfate (SO 4 -2) and sulfite (SO 3 -2) would be sulfic acid and sulfous acid, we actually use more than just the beginning of sulfur in the names of these acids. The same is true for phosphate. H 2 SO 4 H 2 SO 3 H 3 PO 4 Sulfuric acid Sulfurous acid Phosphoric acid

1. Binary acids ALWAYS begin with the prefix hydro 2. Binary acids ALWAYS end with the suffix -ic 3. Acids of oxyanions NEVER begin with hydro 4. If an oxyanion end with –ate, its acid will end with –ic. 5. If an oxyanion end with –ite, its acid will end with –ous.

Name the acids on your notes according to the rules and information you received in class today. hydrobromic acid carbonic acid nitrous acid chromic acid hydrofluoric acid phosphoric acid hydrochloric acid acetic acid

Naming Bases Arrhenius bases are named according to the rules of ionic compounds. We learned these earlier. Na. OH Sodium Hydroxide Name the metal ion Name the anion Sodium Hydroxide Remember that Arrhenius defined bases as hydroxide ion donors. This means that every Arrhenius base will contain the anion hydroxide.

Name the bases on your notes according to the rules and information you received in class today. potassium hydroxide barium hydroxide cesium hydroxide calcium hydroxide lithium hydroxide ammonium hydroxide
- Slides: 23