Names Formulas Nomenclature Anatomy of a Chemical Formula










































- Slides: 51

Names & Formulas (Nomenclature)

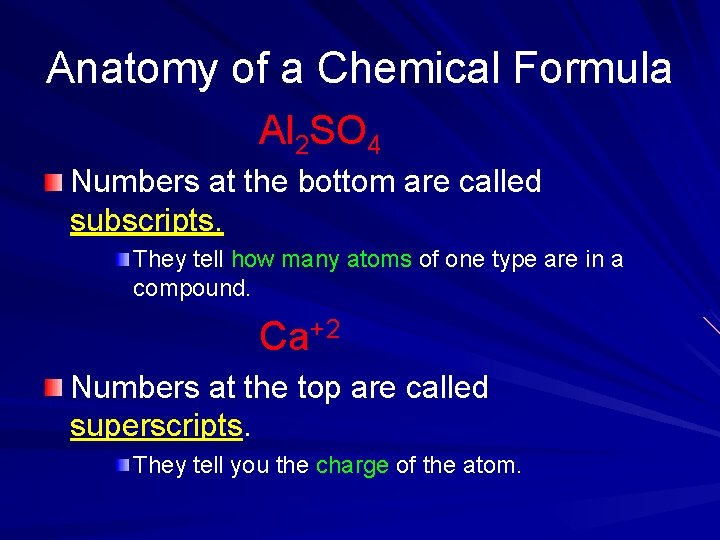
Anatomy of a Chemical Formula Al 2 SO 4 Numbers at the bottom are called subscripts. They tell how many atoms of one type are in a compound. Ca+2 Numbers at the top are called superscripts. They tell you the charge of the atom.

Polyatomic Ions Group of atoms that have an overall charge. You will find both subscripts and superscripts in polyatomic ions. CO 3 -2 carbon = 1 oxygen = 3 overall charge = -2

3 Categories of Compounds 1. Ionic 2. Acids & Bases 3. Molecular

1) Ionic Compounds Contain IONIC bonds. Formed between metals and non-metals. Ions combine in small, whole number ratios. The sum of oxidation numbers will be zero! (they are neutral)

Binary Ionic Compounds All contain two elements. All end in “ide”.

Binary Ionic Compounds To write formula: 1) Write + and - ions. 2) Reduce oxidation numbers, if possible. 3) “Criss-Cross” number only to become subscript for other ion.

Examples… sodium chloride magnesium nitride calcium oxide Na. Cl Mg 3 N 2 Ca. O

Binary Ionic Compounds To Name: 1) Cations (+) retain the name of the element. 2) Anions (-) keep the root name but add “ide” ending.

Examples… Li. F Lithium fluoride Sr. Cl 2 Strontium chloride Al 2 O 3 Aluminum oxide

Multi-Charge Metals Most of the transition metals (and lead and tin) have variable oxidation numbers. (they can have more than one)

Roman numerals are used to indicate charges for these elements. Roman numerals always belong to the metal. (are always positive)

IMPORTANT EXCEPTIONS! Silver always forms a 1+ ion. Zinc always forms a 2+ ion. Cadmium always forms a 2+ ion.

Examples… Iron (II) oxide Fe. O iron(III) oxide Fe 2 O 3 chromium (IV) sulfide Cr. S 2

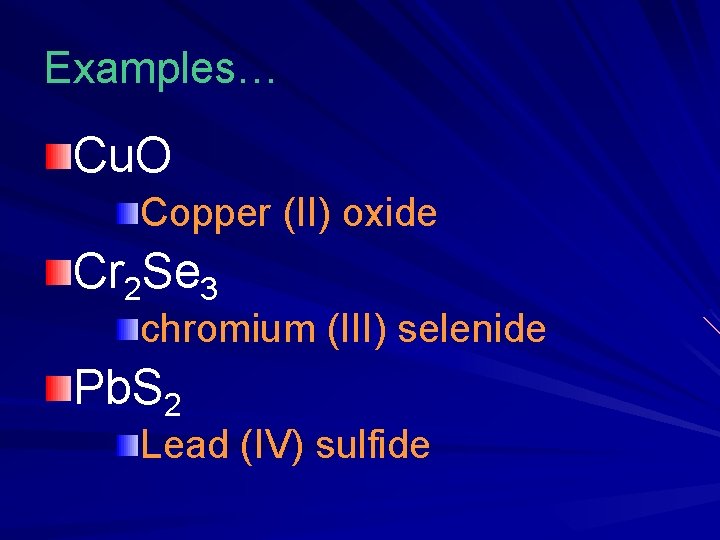
Examples… Cu. O Copper (II) oxide Cr 2 Se 3 chromium (III) selenide Pb. S 2 Lead (IV) sulfide

Ternary Ionic Compounds Contain 3 or more elements. (not binary) Contain at least one Polyatomic Ion. (look on the back of your P. T. )

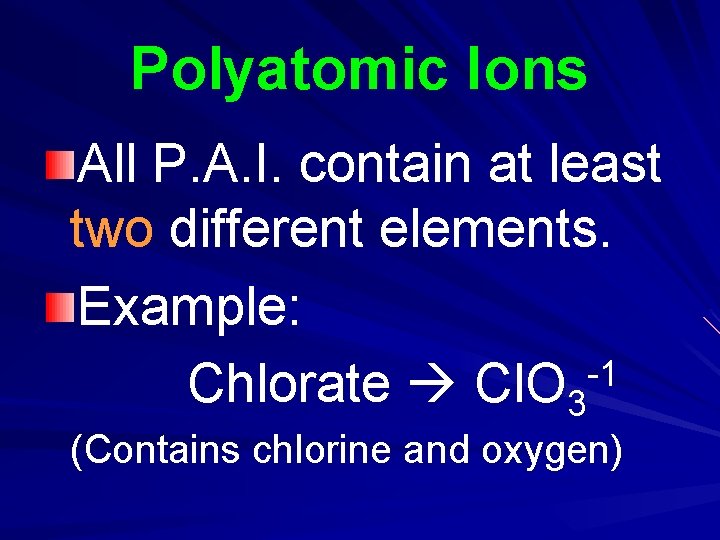
Polyatomic Ions All P. A. I. contain at least two different elements. Example: -1 Chlorate Cl. O 3 (Contains chlorine and oxygen)

To name/write formulas: Use the same rules as binary ionic compounds. Enclose P. A. I. in parenthesis when necessary.

Examples…. . Aluminum phosphate Al. PO 4 Sodium sulfate Na 2 SO 4 Iron (II) bromate Fe(Br. O 3)2

Examples… Ca. CO 3 Calcium carbonate Cu(NO 3)2 Copper (II) nitrate (NH 4)3 N Ammonium nitride

Naming Acids & Bases

2) Acids and Bases: All traditional acids begin with Hydrogen (H). All traditional bases end with Hydroxide (OH).

Acids are easy to recognize because they begin with “H”. Bases are easy to recognize because they end with “OH”.

Bases There are no special rules for naming bases! (they are ionic) EX: Na. OH Sodium hydroxide

Binary Acids Have only two elements. (hydrogen and a nonmetal from the Periodic Table)

To Name: 1) Use the prefix “hydro”. 2) Add the suffix “ic”. Examples: HCl H 3 N hydrochloric acid hydronitric acid

To write formulas: +1 H 1) Start with 2) End with negative (non-metal) ion from periodic table. 3) “Criss-Cross”.

Examples: Hydroiodic acid HI Hydrophosphoric acid H 3 P

Ternary Acids Have at least three elements: (H and a polyatomic ion)

To Name: 1) Use NO “hydro” prefix! 2) Add suffix: if “ate” ion, “ic” suffix if “ite” ion, “ous” suffix

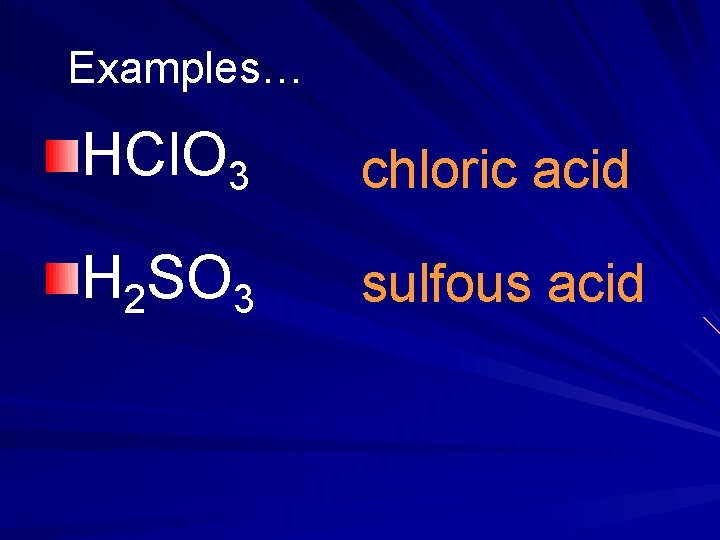
Examples… HCl. O 3 chloric acid H 2 SO 3 sulfous acid

To write formula: 1) 2) +1 H Start with End with negative (polyatomic) ion. 3) “Criss-Cross”.

Examples: perchloric acid HCl. O 4 chlorous acid HCl. O 2

3) Molecular: Used to name Covalently bonded atoms. Made up of non-metals only.

Molecular Compounds

Contain COVALENT bonds. The same elements can combine in various ways. Prefixes are used to tell the difference between them.

We will only learn BINARY molecular compounds. Binary = only contains two different elements. All binary compounds end in “ide”.

Prefixes: 1 = mono 2 = di 3 = tri 4 = tetra 5 = penta 6 = hexa 7 = hepta 8 = octa 9 = nona 10 = deca

To name: Use prefixes on first atom, except for “mono”. Always use prefixes on the last atom. Change ending to “ide”.

Examples… S 2 O 3 disulfur trioxide CO carbon monoxide OF 2 oxygen difluoride

To write formulas: Look at prefixes attached to each element to determine subscript.

Examples… Arsenic pentiodide As. I 5 Carbon ditelluride CTe 2 Diphosphorus trioxide P 2 O 3

Hydrocarbons

Organic (Molecular) compounds that contain only hydrogen and carbon.

Alkanes have single bonds Alkenes have double bonds Alkynes have triple bonds

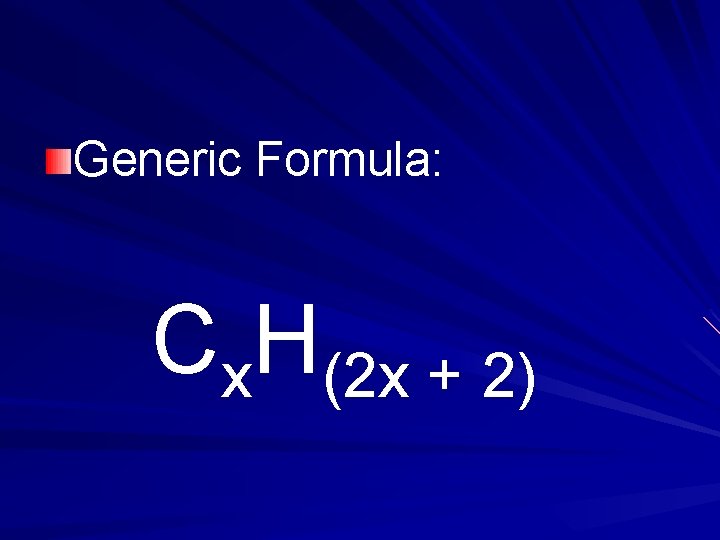
Alkanes carbon chain held together with single bonds.

Generic Formula: Cx. H(2 x + 2)

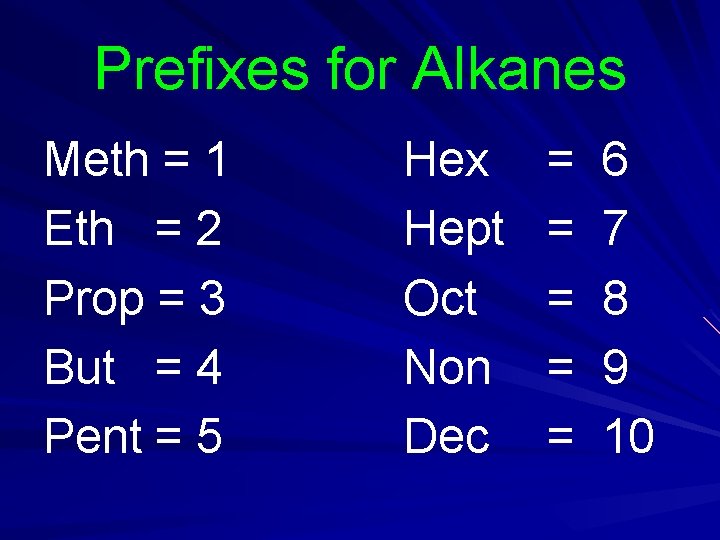
Names Based on number of carbons in molecule (prefixes indicate #) All end in “ane”.

Prefixes for Alkanes Meth = 1 Eth = 2 Prop = 3 But = 4 Pent = 5 Hex Hept Oct Non Dec = = = 6 7 8 9 10

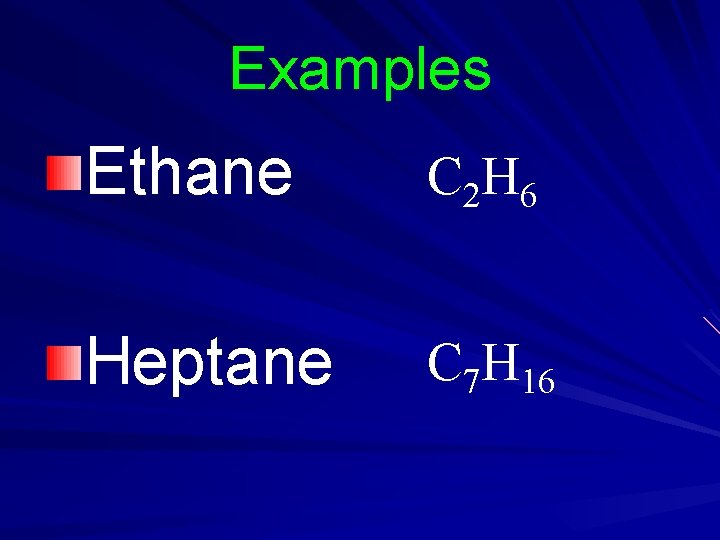
Examples Ethane C 2 H 6 Heptane C 7 H 16

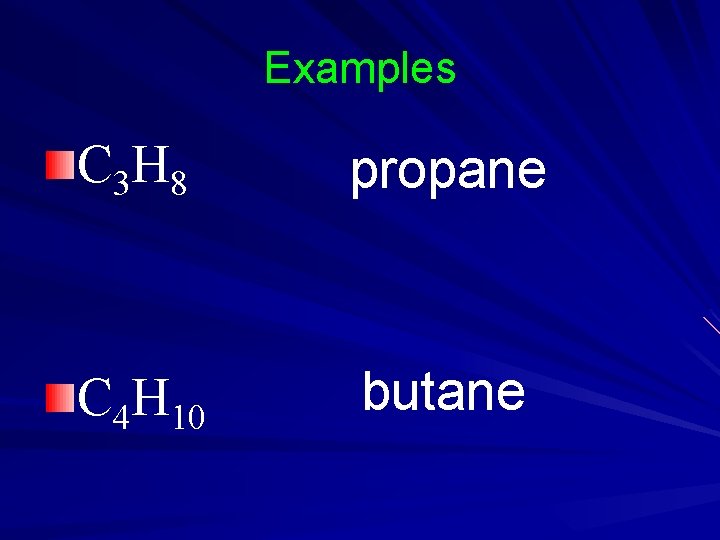
Examples C 3 H 8 propane C 4 H 10 butane